Abstract
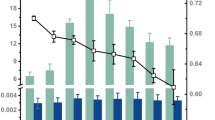
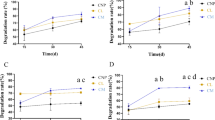
The vertical distribution of microbial biomass, activity, community structure and the mineralization of xenobiotic chemicals was examined in two soil profiles in northern Wisconsin. One profile was impacted by infiltrating wastewater from a laundromat, while the other served as a control. An unconfined aquifer was present 14 meters below the surface at both sites. Biomass and community structure were determined by acridine orange direct counts and measuring concentrations of phospholipid-derived fatty acids (PLFA). Microbial activity was estimated by measuring fluorescein diacetate (FDA) hydrolysis, thymidine incorporation into DNA, and mixed amino acid (MAA) mineralization. Mineralization kinetics of linear alkylbenzene sulfonate (LAS) and linear alcohol ethoxylate (LAE) were determined at each depth. Except for MAA mineralization rates, measures of microbial biomass and activity exhibited similar patterns with depth. PLFA concentration and rates of FDA hydrolysis and thymidine incorporation decreased 10–100 fold below 3 m and then exhibited little variation with depth. Fungal fatty acid markers were found at all depths and represented from 1 to 15% of the total PLFAs. The relative proportion of tuberculostearic acid (TBS), an actinomycete marker, declined with depth and was not detected in the saturated zone. The profile impacted by wastewater exhibited higher levels of PLFA but a lower proportion of TBS than the control profile. This profile also exhibited faster rates of FDA hydrolysis and amino acid mineralization at most depths. LAS was mineralized in the upper 2 m of the vadose zone and in the saturated zone of both profiles. Little or no LAS biodegradation occurred at depths between 2 and 14 m. LAE was mineralized at all depths in both profiles, and the mineralization rate exhibited a similar pattern with depth as biomass and activity measurements. In general, biomass and biodegradative activities were much lower in groundwater than in soil samples obtained from the same depth.
Similar content being viewed by others
References
Beloin RM, Sinclair JL, Ghiorse WC (1988). Distribution and activity of microorganisms in subsurface sediments of a pristine study site in Oklahoma. Microb Ecol 16:85–98
Bone TL, Balkwill DL (1988) Morphological and cultural comparison of microorganisms in surface soil and subsurface sediments at a pristine study site in Oklahoma. Microb Ecol 16: 49–64
Boyer SL, Guin KF, Kelley RM, Mausner ML, Robinson HF, Schmitt TM, Stahl CR, Setzkorn EA (1977) Analytical method for nonionic surfactants in laboratory biodegradation and environmental studies. Environ Sci Technol 11:1167–1171
Colwell FS (1989) Microbiological comparison of subsurface soil and unsaturated subsurface soil from a semiarid high desert. Applied Environ Microbiol 55:2420–2423
Dunlap WJ, McNabb JF, Scalf MR, Cosby RL (1977) Sampling for organic chemicals and microorganisms in the subsurface. EPA-600/2-77-176. US Environmental Protection Agency
Federle TW (1986) Microbial distribution in soil—New techniques. In: Megusar F, Gantar M (eds) Perspectives in microbial ecology, Slovene Society for Microbiology, Ljubljana, pp 493–498
Federle TW (1988) Mineralization of monosubstituted aromatic compounds in unsaturated and saturated subsurface soils. Can J Microbiol 34:1037–1042
Federle TW, Dobbins DC, Thornton-Manning JR, Jones DC (1986) Microbial biomass, activity and community structure in subsurface soils. Ground Water 24:365–374
Federle TW, Pastwa GM (1988) Biodegradation of surfactants in saturated subsurface sediments: A field study. Ground Water 26:761–770
Ghiorse WC, Balkwill, DL (1983) Enumeration and morphological characterization of bacteria indigenous to subsurface environments. Dev Ind Microbiol 24:213–224
Guckert JB, Antworth CB, Nichols PD, White DC (1985) Phospholipid ester-linked fatty acid profiles as reproducible assays for changes in prokaryotic community structure of estuarine sediments. FEMS Microbiol Ecol 31:147–158
Hirsch P, Rades-Rohkohl E (1983) Microbial diversity in a groundwater aquifer in northern Germany. Dev Ind Microbiol 24:183–200
Hobbie JE, Daley RJ, Jasper S (1977) Use of Nucleopore filters for counting bacteria by fluorescence microscopy. Applied Environ Microbiol 33:1225–1228
Kolbel-Boelke J, Anders EM, Nehrkorn A (1988) Microbial communities in saturated groundwater environment II: Diversity of bacterial communities in Pleistocene sand aquifer and their in vitro activities. Microb Ecol 16:31–48
Larson RJ (1984) Kinetic and ecological approaches for predicting biodegradation rates of xenobiotic organic chemicals in natural ecosystems. In: Klug MJ, Reddy CJ (eds) Current perspectives in microbial ecology, American Society for Microbiology, Washington, DC, pp 677–686
Marxsen J (1988) Investigations into the number of respiring bacteria in groundwater from sandy and gravelly deposits. Microbial Ecol 16:65–72
Osburn QW (1986) Analytical methodology for linear alkylbenzene sulfonate in waters and waste. JAOCS 63:257–263
Sinclair JL, Ghiorse WC (1987) Distribution of protozoa in subsurface sediments of a pristine groundwater study site in Oklahoma. Applied Environ Microbiol 53:1157–1163
Smith GA, Nickels JS, Kerger BD, Davis JD, Collins SP, Wilson JT, McNabb JF, White DC (1986) Quantitative characterization of microbial biomass and community structure in subsurface material: A prokaryotic consortium responsive to organic contamination. Can J Microbiol 32:104–111
Thomas JM, Lee MD, Ward CH (1987) Use of ground water in assessment of biodegradation potential in the subsurface. Environ Toxicol Chem 6:607–614
Thorn PM, Ventullo RM (1988) Measurement of bacterial growth rates in subsurface sediments. Microb Ecol 16:3–16
United States Department of Agriculture (1972) Soil survey laboratory methods and procedures for collecting soil samples. Soil Conservation Service. Soil Survey Investigations Report No 1
Webster JJ, Hampton GJ, Wilson JT Ghiorse WC, Leach FR (1984) Determination of microbial cell number in subsurface samples. Ground Water 22:17–25
Werdelmann BW (1984) Tenside in unserer Welte-heute und morgen. Proc World Surfactants Congr II 1:3–21
White DC, Smith GA, Gehron MJ, Parker JH, Findlay RH, Martz RF, Fredrickson HL (1983) The groundwater aquifer microbiota: Biomass, community structure and nutritional status. Dev Ind Microbiol 24:201–210
Wilson JT, McNabb JF, Balkwill DL, Ghiorse WC (1983) Enumeration and characterization of bacteria indigenous to a shallow water-table aquifer, Ground Water 21:134–142
Wilson JT, McNabb JF, Wilson BH, Noonan MJ (1983) Biotransformation of selected organic pollutants in ground water. Dev Ind Microbiol 24:225–233
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Federle, T.W., Ventullo, R.M. & White, D.C. Spatial distribution of microbial biomass, activity, community structure, and the biodegradation of linear alkylbenzene sulfonate (LAS) and linear alcohol ethoxylate (LAE) in the subsurface. Microb Ecol 20, 297–313 (1990). https://doi.org/10.1007/BF02543885
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02543885