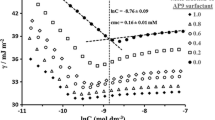
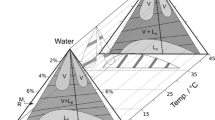
Abstract
Recent phase studies of several surfactant-water systems, using new or refined methods, have revealed significant errors in earlier phase diagrams. These diagrams had been determined largely using methods based on the isoplethal phase studies principle. This principle has inherent limitations which do not exist in isothermal methods.
Isothermal nuclear magnetic resonance and refined calorimetric methods have been extensively used in recent surfactant phase studies. Methods based on the new lyotrope gradient (swelling) principle show great promise as a means of improving the efficiency and quality of surfactant phase studies.
Similar content being viewed by others
References
McBain, J.W.,Colloid Chemistry, edited by J. Alexander, The Chemical Catalog Co., Inc., New York, 1926, pp. 137–164.
Roozeboom, H.W. Bakhuis, and F.A.H. Schreinemakers, eds..Die Heterogene Gleichgewischte Vols. I–III, F. Vieweg u. Sohn, Braunschweig, 1901–1918.
Hill, A.E.,J. Am. Chem. Soc. 45:1143 (1923).
Lang, J.C., Jr., and B. Widom,Physica 81A:190 (1975).
Purdon, F.F., and V.W. Slater,Aqueous Solution and the Phase Diagram, Edward Arnold & Co., London, 1946.
Lang, J.C., and R.D. Morgan,J. Chem. Phys. 73:5849 (1980).
Alexejew, W.,J. Prakt. Chem. 25:518 (1882).
Laughlin, R.G.,J. Coll. Interface Sci. 55:239 (1976).
Kekicheff, P., and B. Cabane,J. Phys. 48:1571 (1987).
Kekicheff P., C. Grabielle-Madelmont, and M. Ollivon,J. Coll. Int. Sci., 131:112 (1989).
Kekicheff, P.J. Coll. Int. Sci., 131:133 (1989).
Laughlin, R.G., R.L. Munyon, Y.-C. Fu, and A.J. Fehl,J. Phys. Chem. 94:2546 (1990).
Alberty, R.A., and F. Daniels,Physical Chemistry, John Wiley & Sons, New York, 1979.
Laughlin, R.G., inFood Emulsion and Foams: Theory and Practice, AIChE Symposium Series, No. 277, Vol. 86, 1990, pp. 7–15.
Sjoblom, J., P. Stenius and I. Danielsson,Nonionic Surfactants. Physical Chemistry, edited by Martin J. Schick, Marcel Dekker, Inc., New York, 1987, pp. 369–434.
Laughlin, R.G., and R.L. Munyon,J. Phys. Chem. 91:3299 (1987).
Ulmius, J., H. Wennerstrom, G. Lindblom and G. Arvidson,Biochemistry 16:5742 (1977).
Lindblom, G., and L. Rilfors,Biochim. Biophys. Acta 988:221 (1989).
Kessis, J.-J.,C.R. Acad. Sc. Paris, Series C 270:1 (1970).
Caffrey, M.,Biophys. J. 51:444a (1987).
Blackmore, E.S., and G.J.T. Tiddy,J. Chem. Soc. Faraday Trans. 2 84:1115 (1988).
Madelmont, C., and R. Perron,Bull. Soc Chim. France, 3259 (1973).
Ibid. Madelmont, C., and R. Perron,Bull. Soc. Chim. France, 3263 (1973).
Ibid. Madelmont, C., and R. Perron,Bull. Soc. Chim. France 1795 (1974).
Ibid. Madelmont, C., and R. Perron,Bull. Soc. Chim. France, 1799 (1974).
Ibid. Madelmont, C., and R. Perron,Bull. Soc. Chim. France, 3425 (1974).
Ibid. Madelmont, C., and R. Perron,Bull. Soc. Chim. France, 3430 (1974).
Madelmont, C., and R. Perron,Coll. Poly. Sci. 254:6581 (1976).
Rosevear, F.B.,J. Soc. Cos. Chem. 19:581 (1968).
Rendall, K., G.J.T. Tiddy and M.A. Trevethan,J. Chem. Soc., Faraday Trans 1 79:637 (1983).
Kekicheff, P., and G.J.T. Tiddy,J. Phys. Chem. 93:2520 (1989).
Kuneida, H., and K. ShinodaIbid. 82:1710 (1978).
Gault, J.D., M.A. Leite, M.R. Rizzatti and H. Gallardo,J. Coll. Int. Sci. 122:587 (1988).
Evans, D.F., and B.W. Ninham,J. Phys. Chem. 90:226 (1986).
Vincent, J.M., and A. Skoulios,Acta Cryst. 20:432 (1966).
Cev, G., and D. Marsh,Polar Lipids Bilayer: Physical Principles and Models, John Wiley & Sons, Inc., New York, 1987.
Author information
Authors and Affiliations
About this article
Cite this article
Laughlin, R.G. Status of aqueous surfactant phase science. J Am Oil Chem Soc 67, 705–710 (1990). https://doi.org/10.1007/BF02540476
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02540476