Abstract
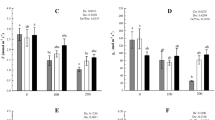
Squash, like other Cucurbitaceae, have unique sterol profiles that offer an excellent opportunity to examine the relationship between sterol biosynthesis and plant growth. To determine the effect of sterol biosynthesis inhibition on squash growth, Cucurbita maxima seedlings with and without cotyledons were subjected to increasing concentrations of the cycloartenol synthase (EC 5.4.99.8) inhibitor 3β-(2-diethylaminoethoxy) androstenone (U18666A). Inhibition of shoot growth was concentration-dependent (from 0, 2, 5, 10, and 20 μM); plants with intact cotyledons grew to 26.4, 23.7, 21.6, 20.0, and 15.6 cm, respectively, at the above inhibitor concentrations, compared to 25.5, 19.4, 17.0, 12.0, and 11 cm for plants with severed cotyledons. In plants with severed cotyledons, 10 and 20 μM U18666A caused rapid necrosis of the first two, newly emerged, primary leaves, and halted new leaf formation. Secondary root formation was initially affected at all inhibitor concentrations regardless of whether cotyledons were present or not. Vegetative tissue showed a decrease in the accumulation of the major squash sterol, 7, 22-stigmastadienol, accompanied by increased accumulation of minor sterol components. Sterol profiles in cotyledons were unaltered. The data show that sterols are crucial for maintaining plant growth and viability, but do not address the cotyledonary effect on growth with respect to sterol biosynthesis.
Similar content being viewed by others
Abbreviations
- FID:
-
flame-ionization detection
- GC:
-
gas chromatography
- HMG-CoA:
-
hydroxymethglutaryl CoA
- TMSE:
-
trimethylsilyl ether
- U18666A:
-
3β-(2-diethylaminoethoxy)androstenone
References
Bach, T.J. (1986)Lipids 21, 82–88.
Yates, P.J., Lenton, J.R., and Goad, L.J. (1993)Pestic. Sci. 39, 257–265.
Khalil, I.A., and Mercer, E.I. (1990)Phytochemistry 29, 417–424.
Rahier, A., Taton, M., Bouvier-Nave, P., Schmitt, P., Benveniste, P., Schuber, F., Narula, A.S., Cattel, L., Anding, C., and Place, P. (1986)Lipids 21, 52–62.
Cattel, L., Ceruti, M., Delprino, L., Balliano, G., Duriatti, A., and Bouvier-Nave, P. (1986)Lipids 21, 31–38.
Burden, R.S., Cooke, D.T., and Carter, G.A. (1989)Phytochemistry 28, 1791–1880.
Mercer, E.I. (1991)Lipids 26, 584–597.
Mercer, E.I. (1993)Prog. Lipid Res. 32, 357–416.
Bach, T.J., and Lichtenthaler, H.K. (1987)Ecology and Metabolism of Plant Lipids (Fuller, G., and Nes, W.D., eds.) Vol. 325, pp. 109–139, American Chemical Society Washington, DC.
Fenner, G.P., Patterson, G.W., and Lusby, W.R. (1989)Lipids 24, 271–277.
Garg, V.K., and Nes, W.R. (1985)Lipids 20, 876–883.
Sucrow, W., Slopianka, M., and Kircher, H.W. (1970)Phytochemistry 15, 1533–1535.
Fenner, G.P., and Patterson, G.W. (1992)Phytochemistry 31, 73–75.
Holden, M.J., and Patterson, G.W. (1982)Lipids 17, 215–219.
Thompson, Jr., R.C., Patterson, G.W., Thompson, M.J., and Slover, H.T. (1981)Lipids 16, 694–699.
Haughan, P.A., Burden, R.S., Lenton, J.R., and Goad, L.J. (1989)Phytochemistry 28, 781–787.
Kutschera, U. (1992)J. Plant Physiol. 140, 319–323.
Ceccarelli, N., and Lorenzi, R. (1992)J. Plant. Physiol. 140, 190–194.
Rodriguez, R.L., Taylor, F.R., and Parks, L.W. (1982)Biochem. Biophys. Res. Commun. 106, 435–448.
Fenner, G.P., Patterson, G.W., and Koines, P.M. (1986)Lipids 21, 48–51.
Heupal, R.C., Sauvaire, Y., Le, Ph., Parish, E.J., and Nes, W.D. (1986)Lipids 21, 69–75.
Kalinowska, M., Nes, W.R., Crumley, I-G., and Nes, W.D. (1990)Phytochemistry 29, 3427–3434.
Author information
Authors and Affiliations
About this article
Cite this article
Fenner, G.P., Raphiou, I. Growth ofCururbita maxima L. plants in the presence of the cycloartenol synthase inhibitor U18666A1 . Lipids 30, 253–256 (1995). https://doi.org/10.1007/BF02537829
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02537829