Abstract
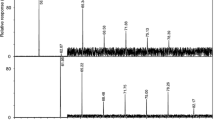
Arange of analytical techniques was used to investigate the composition of the steryl fatty acyl esters in a cell suspension culture of celery (Apium graveolens). Gas chromatography (GC) and GC-mass spectrometry (GC-MS), using electron ionization (EI) and negative ion chemical ionization (NICI), were employed to characterize the intact steryl esters. Assignments were supported by analysis of the sterol and fatty acid moieties released from the intact molecular species by alkaline hydrolysis. A selectivity for sterol esterification was noted, with the major free sterol, stigmasterol, occurring only in a very small amount in the esterified form. Instead, the precursors to Δ5-phytosterols, particularly cycloartenol, predominated in the ester fraction. The pentacyclic triterpene, β-amyrin, was also found as the palmitate and linoleate esters. Changes in composition and abundance of the steryl esters during the different growth phases of a celery cell suspension culture were investigated. The total amount of esterified sterols exceeded that of free sterols throughout the growth cycle. The changes observed during growth highlighted differences between the esters of precursor sterols and those of the 4-desmethyl-sterols, and it is postulated that the various steryl esters perform different functions in cell metabolism.
Similar content being viewed by others
Abbreviations
- EI:
-
electron impact ionization
- FS:
-
free sterols
- GC:
-
gas chromatography
- M+ :
-
molecular ion
- MS:
-
mass spectrometry
- NICI:
-
negative ion chemical ionization
- SAM:
-
S-adenosyl-L-methionine
- SE:
-
steryl esters
References
Goad, L.J., Zimowski, J., Evershed, R.P. and Male, V.L. (1987)The Metabolism, Structure and Function of Plant Lipids (Stumpf, P.K., Mudd, J.B., and Nes, W.D., eds.) pp. 95–120, Plenum Press, New York and London.
Bush, P.B., and Grunwald, C. (1972)Plant Physiol. 50, 69–72.
Kintia, P.K., and Wojciechowski, Z.A. (1974)Phytochemistry 13, 2235–2238.
Garcia, R.E., and Mudd, J.B. (1978)Plant Physiol. 61, 354–356.
Duperon, R., Thiersault, M., and Duperon, P. (1984)Phytochemistry 23, 743–746.
Heupel, R.C., and Nes, W.D. (1984)J. Nat. Prod. 47, 292–299.
Kalinowska, M., and Wojciechowski, Z.A. (1984)Phytochemistry 23, 2485–2488.
Takaoka, D., Matsuo, A., and Hayashi, S. (1987)Phytochemistry 26, 429–432.
Huang, L.S., and Grunwald, C. (1988)Phytochemistry 27, 2049–2053.
Kemp, R.J., and Mercer, E.I. (1968)Biochem. J. 110, 111–118.
Kemp, R.J., and Mercer, E.I. (1968)Biochem. J. 110, 119–125.
Hartmann, M.A., Ferne, M., Gigot, C., Brandt, R., and Benveniste, P. (1973)Physiol. Veg. 11, 209–230.
Janiszowska, W., and Kasprzyk, Z. (1977)Phytochemistry 16, 473–476.
Garcia, R.E., and Mudd, J.B. (1978)Plant Physiol. 61, 357–360.
Duperon, P. (1971)Physiol. Veg. 9, 373–399.
Kasprzyk, Z., Pyrek, J., and Turowska, G. (1968)Acta Biochimica Polonica 15, 149–158.
Kemp, R.J., Goad, L.J., and Mercer, E.I. (1967)Phytochemistry 6, 1609–1615.
Atallah, A.M., Aexel, R.T., Ramsey, R.B., Threlkeld, S., and Nicholas, H.J. (1975)Phytochemistry 14, 1927–1932.
Atallah, A.M., and Nicholas, H.J. (1974)Lipids 9, 613–622.
Kemp, R.J., Hammam, A.S.A., Goad, L.J., and Goodwin, T.W. (1968)Phytochemistry 7, 447–450.
Goad, L.J. (1983)Biochem. Soc. Trans. 11, 548–552.
Katz, S.S., and Small, D.M. (1980)J. Biol. Chem. 255, 9753–9759.
Rapp, J.H., Connor, W.E., Lin, D.S., Inahara, T., and Porter, J.M. (1983)J. Lipid Res. 24, 1329–1335.
Hughes, M.A., and Goad, L.J. (1983)Biochem. Soc. Trans. 11, 588–589.
Whitaker, B.D. (1988)Phytochemistry 27, 3411–3416.
Lalaguna, F., and Agudo, M. (1989)Phytochemistry 28, 2059–2062.
Evershed, R.P., Prescott, M.C., Spooner, N., and Goad, L.J. (1989)Steroids 53, 288–309.
Evershed, R.P., Male, V.L., and Goad, L.J. (1987)J. Chromatog. 400, 187–205.
Kates, M. (1972)Techniques in Lipidology (Work, T.S., and Work, E., eds.) p. 573, Elsevier, Holland, New York.
Evershed, R.P., and Goad, L.J. (1987)Biomed. Env. Mass Spectrom. 14, 131–140.
Wakeham, S.G., and Frew, N.M. (1982)Lipids 17, 831–843.
Lusby, W.R., Thompson, M.J., and Kochansky, J. (1984)Lipids 19, 888–901.
Haughan, P.A., Lenton, J.R., and Goad, L.J. (1988)Phytochemistry 27, 2491–2500.
Nagai, J., Kawamura, S., and Katsuki, H. (1977)J. Biochem. 81, 1665–1673.
Goad, L.J., Haughan, P.A., and Lenton, J.R. (1988) inPlant Lipids: Targets for Manipulation (Pinfield, N.J., and Stobart, A.K., eds.) pp. 91–105.
Taylor, F.R., and Parks, L.W. (1981)J. Biol. Chem. 256, 13048–13054.
Bailey, R.B., and Parks, L.W. (1975)J. Bacteriol. 124, 606–612.
Taylor, F.R., and Parks, L.W. (1978)J. Bacteriol. 136, 531–537.
Yates, P.J., Haughan, P.A., Lenton, J.R., and Goad, L.J. (1990) inPlant Lipid Biochemistry-Structure and Utilisation (Quinn, P.J., and Harwood, J., eds.) pp. 341–343, Portland Press, London.
Lorenz, R.T., and Parks, L.W. (1990)Antimicrob. Agents & Chemotherapy 34, 1660–1665.
Baisted, D.J. (1971)Biochem. J. 124, 375–383.
Author information
Authors and Affiliations
About this article
Cite this article
Dyas, L., Prescott, M.C., Evershed, R.P. et al. Steryl esters in a cell suspension culture of celery (Apium graveolens). Lipids 26, 536–541 (1991). https://doi.org/10.1007/BF02536600
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02536600