Abstract
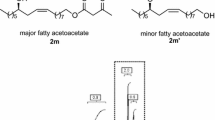
A novel 1-pyrroline fatty acid ester isomer [viz. 8-(5-hexyl-1-pyrrolin-2-yl)octanoate] has been synthesized from methyl ricinoleate by two routes with an overall yield of 42 and 30%, respectively. Most of the reactions are carried out under concomitant ultrasonic irradiation (20 KHz,ca. 53 watts/cm2). Under such a reaction condition, the reaction time is considerably shortened, and product yields are high. Dehydrobromination under concomitant ultrasonic irradiation of methyl 9,10-dibromo-12-hydroxyoctadecanoate with KOH in EtOH furnishes methyl 12-hydroxy-9-octadecynoate (66%) within 15 min. Hydration of the latter under ultrasound with mercury(II)acetate in aqueous tetrahydrofuran yields exclusively methyl 12-hydroxy-9-oxo-octadecanoate (95%) in 30 min. The hydroxy group in the latter compound is transformed to the azido functionvia the mesylate, and treatment of the azido-oxo intermediate (methyl 12-azido-9-oxooctadecanoate) with Ph3P under ultrasonic irradiation furnishes the requisite 1-pyrroline fatty acid ester (77%). The same azido-oxo intermediate has also been obtained by the oxidation of methyl 12-azido-9-cis-octadecenoate using benzoquinone and a catalytic amount of Pd(II)chloride in aqueous tetrahydrofuran under concomitant ultrasonic irradiation (90 min) to give the product in 45% yield. The latter reaction does not take place even under prolonged silent stirring of the reaction mixture.
Similar content being viewed by others
Abbreviations
- ECL:
-
equivalent chain length
- GLC:
-
gas-liquid chromatography
- IR:
-
infrared
- MS:
-
mass spectrometry
- NMR:
-
nuclear magnetic resonance spectroscopy
- Rf :
-
retardation factor
- THF:
-
tetrahydrofuran
- TLC:
-
thin-layer chromatography
References
Pedder, D.J., Fales, H.M., Jaouni, T., Blum, M., MacConnell, J., and Crewe, R.M. (1976)Tetrahedron 32, 2275–2279.
Jones, T.H., Blum, M.S., and Fales, H.M. (1982)Tetrahedron 39, 1949–1958.
Caro, M.R., Derbes, V.J., and Jung, R. (1957)Arch. Dermatol. 75, 475–478.
Androuny, G.A., Derbes, V.J., and Jung, R.C. (1969)Science 130, 449.
Jones, T.H., Blum, M.S., and Fales, H.M. (1979)Tetrahedron Lett. 12, 1031–1034.
Jones, T.H., Franko, J.B., Blum, M.S., and Fales, H.M. (1980)Tetrahedron Lett. 21, 789–792.
Shiosaki, K., and Rapoport, H. (1985)J. Org. Chem. 50, 1229–1239.
Fraser, R.R., and Passannanti, S. (1976)Synthesis, 540–541.
Arseniyadis, S., Huang, P.Q., Piveteau, D., and Husson, H.P. (1988)Tetrahedron 44, 2457–2470.
Lie Ken Jie, M.S.F., and Syed-Rahmatullah, M.S.K. (1991)J. Chem. Soc. Perkin Trans. 1, 421–424.
Gunstone, F.D. (1954)J. Chem. Soc., 1611–1616.
Miwa, T.K., Mikolajczak, K.L., Earle, F.R., and Wolff, I.A. (1960)Anal. Chem. 32, 1739–1742.
Gunstone, F.D., and Perera, B.S. (1973)Chem. Phys. Lipids 11, 43–65.
Crombie, L., and Jacklin, A.G. (1955)J. Chem. Soc., 1740–1748.
Lambert, P.H., Vaultier, M., and Carrie, R. (1982)J. Chem. Soc. Chem. Commun., 1224–1225.
Lie Ken Jie, M.S.F., Lam, W.L.K., and Lao, H.B. (1989)J. Chem. Soc. Perkin Trans. 1, 1–11.
Author information
Authors and Affiliations
About this article
Cite this article
Lie Ken Jie, M.S.F., Syed-Rahmatullah, M.S.K., Lam, C.K. et al. Ultrasound in fatty acid chemistry: Synthesis of a 1-pyrroline fatty acid ester isomer from methyl ricinoleate. Lipids 29, 889–892 (1994). https://doi.org/10.1007/BF02536258
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02536258