Abstract
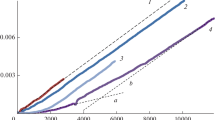
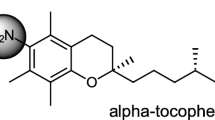
The reaction of α-tocopherol (α-T) with superoxide anion (O −2 ) in both dry acetonitrile and in aqueous acetonitrile solution is described. The O −2 was generated by the electrochemical reduction of molecular oxygen in acetonitrile, using tetrabutylammonium bromide as an electrolyte. α-T was reacted with O −2 either in dry acetonitrile or in a 10% aqueous acetonitrile solution. In dry acetonitrile, α-T was oxidized to a very unstable primary intermediate, which was further oxidized to a secondary, more stable intermediate. The formation of the secondary intermediate depended upon the presence of molecular oxygen. This intermediate readily converted into two compounds in equimolar amounts (designated A and B). The primary, very unstable intermediate was readily reduced again to α-T by treatment with LiAlH4 or ascorbic acid. However, the secondary intermediate or the stable oxidation products could not be reduced to α-T. In the 10% aqueous acetonitrile, α-T was oxidized to α-tocopheryl quinone, α-tocopherol dimer and α-tocopherol dihydroxy dimer, and an unknown compound. In the aqueous medium, no intermediates were formed by the action of O −2 . The results of this study indicate that the reaction of α-T with O −2 under aprotic conditions is different from that observed under protic conditions.
Similar content being viewed by others
Abbreviations
- α-T:
-
α-tocopherol
- D:
-
α-T dimer
- DHD:
-
α-T dihydroxy dimer
- HPLC:
-
high-performance liquid chromatography
- O −2 :
-
superoxide anion
- O.D.:
-
optical density
- TBAB:
-
tetrabutylammonium bromide
- TQ:
-
α-tocopheryl quinone
References
Babior, B.M. (1982)Can. J. Physiol. Pharmacol. 60, 1353–1358.
Korycka-Dahl, M., and Richardson, T. (1980)J. Dairy Sci. 63, 1181–1198.
Boyer, P.D. (1951)J. Am. Chem. Soc. 73, 733–735.
Gotoh, T., and Shikama, K., (1976)J. Biochem. 80, 397–399.
Fee, J.A. (1980) inBiological and Clinical Aspects of Superoxide and Superoxide Dismutase (Bannister, W.H., and Bannister, J.V., eds.) pp. 41–48, Elsevier/North Holland, Inc., New York.
Matsuo, M., Matsumoto, S., and Iitaka, Y. (1981),Tetrahedron Lett., 3649–3652.
Matsuo, M., Matsumoto, S., Iitaka, Y., Hanaki, A., and Ozawa, T. (1979),J.C.S. Chem. Commun., 105–106.
Nanni, E.J., Stallings, M.D., and Sawyer, D.T., (1980)J. Am. Chem. Soc. 102, 4481–4485.
Nishikimi, M., and Machlin, L.J. (1975)Arch. Biochem. Biophys. 170, 684–689.
Ozawa, T., and Hanaki, A. (1983)Biochem. Int. 6, 685–692.
Eggitt, P.W.R., and Norris, F.W. (1956)J. Sci. Food Agr. 7, 493–511.
Csallany, A. Saari, Chiu, M., and Draper, H.H. (1970)Lipids 5, 63–70.
Ha, Y.L., and Csallany, A. Saari (1988)Lipids 23, 359–361.
Fee, J.A., and Hildenbrand, P.G. (1974)FEBS Lett., 39, 79–82.
Csallany, A. Saari, and Draper, H.H. (1963)Arch. Biochem. Biophys. 100, 335–337.
Ozawa, T., and Hanaki, A. (1981)Chem. Pharm. Bull. 29, 926–928.
Matsumoto, S., Matsuo, M., and Iitaka, Y. (1986)J. Org. Chem. 51, 1435–1440.
Matsumoto, S., and Matsuo, M. (1977)Tetrahedron Lett., 1999–2000.
Ozawa, T., and Hanaki, A. (1985)Biochem. Biophys. Res. Commun. 126, 873–878.
Nishikimi, M. (1975)Arch. Biochem. Biophys. 166, 273–275.
DiGuiseppi, J., and Fridovich, I. (1984)CRC Crit. Rev. Toxicol. 12, 315–341.
Krinsky, N.I. (1979) inSinglet Oxygen. (Wasserman, H.H., and Murray, R.W., eds.) pp. 597–641. Academic Press, New York.
Smith, L.I., Spillane, L.J., and Kolthoff, I.M. (1942)J. Am. Chem. Soc. 64, 447–451.
Matsuo, M., and Matsumoto, S. (1983)Lipids 18, 81–86.
Nishinaga, A., Itahara, T., Shimizu T., and Matsuura, T. (1978)J. Am. Chem. Soc. 100, 1820–1825.
Cohen, G., and Sinet, P.M. (1981) inOxygen and Oxy-Radicals in Chemistry and Biology (Rodgers, M.A.J., and Powers, E.L., eds.) pp. 45–54, Academic Press, New York.
Khan, A.U. (1977)J. Am. Chem. Soc. 99, 370–371.
Pederson, T.C., and Aust, S.D. (1973)Biochem. Biophys. Res. Commun. 52, 1071–1078.
Winterle, J., Dulin, D., and Mill, T. (1984)J. Org. Chem. 49, 491–495.
Grams, G.W., and Inglett, G.E. (1972)Lipids 7, 442–444.
Grams, G.W., Eskins, K., and Inglett, G.E. (1972)J. Am. Chem. Soc. 94, 866–868.
Author information
Authors and Affiliations
About this article
Cite this article
Csallany, A.S., Ha, Y.L. α-tocopherol oxidation mediated by superoxide anion (O −2 ) I. Reactions in aprotic and protic conditions. Lipids 27, 195–200 (1992). https://doi.org/10.1007/BF02536178
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02536178