Abstract
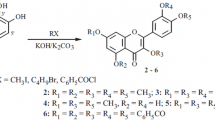
In order to undertake a quantitative study by high-performance liquid chromatography of the rate of oxidation of 2,2,5,7,8-pentamethyl-6-chromanol (1), the model compound of α-tocopherol, a number of potential products were required as standards. Among these compounds were 2,2,7,8-tetramethylchroman-5,6-dione (10) and 2,2,7-trimethyl-6-hydroxychroman-5,8-dione (17), the model compounds of tocored and tocopurple, respectively. Attempts to synthesize 10 and 17 led to the isolation of 8-hydroxymethyl-2,2,7-trimethylchroman-5,6-dione (14) and 1,2-bis(2,2,7-trimethylchroman-5,6-dione-8-)ethane (19) a dimer of 10. Purification by thin-layer chromatography of the spirodimer (20) of 1 resulted in an acid-catalyzed decomposition to 1-(2,2,7,8-tetramethyl-6-chromanol-5-)2-[2-(3-methyl-3-hydroxybutyl)-5,6-dimethyl-1,4-benzoquinone-3-]ethane (23), a new chromanol-quinone dimer.
Similar content being viewed by others
Abbreviations
- E.I.:
-
electron impact
- Fo :
-
observed structure amplitude
- HPLC:
-
high-performance liquid chromatography
- IR:
-
infrared
- Io :
-
observed intensity
- MS:
-
mass spectrum
- NMR:
-
nuclear magnetic resonance
- σ:
-
standard deviation
- θ:
-
diffraction angle
- TLC:
-
thin-layer chromatography
References
Emmerie, A., and Engel, C. (1938)Rec. Trav. Chim. Pays Bas 57, 1351–1355.
Furter, H., and Meyer, R. (1939)Helv. Chim. Acta 22, 240–250.
Smith, L.I., Ungnade, H.E., Hoehn, H., and Wawzonek, S. (1939)J. Org. Chem. 4, 311–317.
Skinner, W.A., and Alaupovic, P. (1963)J. Org. Chem. 28, 2854–2858.
Skinner, W.A., and Parkhurst, R.M. (1964)J. Org. Chem. 29, 3601–3603.
Nakamura, T., and Kijima, S. (1972)Chem. Pharm. Bull. 20, 1681–1686.
Fujimaki, M., Kanamaru, K., Kurata, T., and Igarashi, O. (1970)Ag. Biol. Chem. 34, 1781–1787.
Sumarno, M., Atkinson, E., Suarna, C., Saunders, J.K., Cole, E.R., and Southwell-Keely, P.T. (1987)Biochim. Biophys. Acta 920, 247–250.
Ibers, J.A., and Hamilton, W.C. (eds.) (1974)International Tables for X-Ray Crystallography, Vol. 4 pp. 71–151, Kynoch Press, Birmingham.
Main, P. (1980)MULTAN80, University of York, York.
Busing, W.R., Martin, K.O., and Levy, H.A. (1962)ORFLS, Oak Ridge National Laboratory, Oak Ridge, TN.
Rae, A.D. (1988)RAELS, A Comprehensive Constrained Least Squares Refinement Program, University of New South Wales, Sydney.
Johnson, C.K. (1976)ORTEPII, Oak Ridge National Laboratory, Oak Ridge, TN.
Suarna, C., Craig, D.C., Cross, K.J., and Southwell-Keely, P.T. (1988)J. Org. Chem. 53, 1281–1284.
Skinner, W.A., and Parkhurst, R.M. (1966)J. Org. Chem. 31, 1248–1251.
Nilsson, J.L.G., Branstad, J.O., and Sievertsson, H. (1968)Acta Pharm. Suec. 5, 509–516.
Bolon, D.A. (1970)J. Org. Chem. 35, 715–719.
Suarna, C., Sumarno, M., Nelson, D., and Southwell-Keely, P.T. (1988)Lipids 23, 1129–1131.
Dean, F.M., Hindley, K.B., Houghton, L.E., and Robinson, M.L. (1976)J. Chem. Soc. Perkin I, 600–604.
Horspool, W.M., Smith, P.I., and Tedder, J.M. (1972)J. Chem. Soc. Perkin I, 1024–1030.
Turner, A.B. (1964)Quart. Rev. 28, 347–360.
Nelan, D.R., and Robeson, C.D. (1962)J. Am. Chem. Soc. 84, 2963–2965.
Sykes, P. (1986)A Guidebook to Mechanism in Organic Chemistry, 6th edn., pp. 240–241, Longman, London and New York.
Durckheimer, W., and Cohen, L.A. (1962)Biochem. Biophys. Res. Commun. 9, 262–265.
Author information
Authors and Affiliations
About this article
Cite this article
Suarna, C., Baca, M., Craig, D.C. et al. Further oxidation products of 2,2,5,7,8-pentamethyl-6-chromanol. Lipids 26, 847–852 (1991). https://doi.org/10.1007/BF02536168
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02536168