Abstract
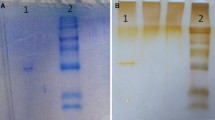
Acetyl-CoA carboxylase is the pivotal enzyme in the de novo synthesis of fatty acids and is the only carboxylase with a biotin-containing subunit greater than 200,000 daltons. The biotin moiety is covalently linked to the active site and has a high affinity (Kd=10−15 M) for the protein avidin. This relationship has been used in previous studies to identify acetyl-CoA carboxylase isolated from mammalian species. However, acetyl-CoA carboxylase has not been isolated and characterized in a poikilothermic species such as the rainbow trout. The present study describes the isolation and identification of acetyl-CoA carboxylase in the cytosol of rainbow trout (Salmo gairdneri) liver. The enzyme was isolated using two distinct procedures—polyethylene glycol precipitation and avidin-Sepharose affinity chromatography. Identification of the isolated protein as acetyl-CoA carboxylase was made by the following: (1) sodium dodecyl sulfate-polyacrylamide gel electrophoresis; (2) avidin binding; (3) in vivo labeling with [14C]biotin; and (4) acetyl-CoA carboxylase-specific activity. The subunit molecular weight of the major protein was 230,000 daltons ±3.3%. This protein was shown to bind avidin (Mr=16,600) prior to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, indicating the presence of biotin. In addition, protein isolated from fish that had previously received intraperitoneal injections of [14C]biotin, showed the majority of radioactivity associated with the 230,000 dalton protein. The polyethylene glycol precipitation yielded 200 μg protein (4.4 μg/g liver), with a specific activity of 5 nmol malonyl-CoA/min/mg protein, whereas avidin affinity chromatography yielded 1.75±1.1 mg protein (9.0 μg/g liver), with a specific activity of 1.37±0.18 μmol malonyl-CoA/min/mg protein. The enzyme was citrate dependent showing maximum activity between 10 and 20 mM. Acetyl-CoA carboxylase-specific activity decreased by 50% in the presence of 0.2 M NaCl. These findings suggest that the major protein (Mr=230,000) purified from rainbow trout liver is acetyl-CoA carboxylase with enzyme characteristics comparable to mammalian acetyl-CoA carboxylase.
Similar content being viewed by others
Abbreviations
- ACC:
-
Acetyl-CoA carboxylase
- CPFA:
-
cyclopropenoid fatty acid
- PEG:
-
polyethylene glycol precipitation
- SDS-PAGE:
-
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
References
Wakil, S.J. (1958)J. Am. Chem. Soc. 80, 6465–6466.
Wakil, S.J. (1958)Biochim. Biophys. Acta 29, 225–226.
Wood, H.G., and Barden, R.E. (1977)Ann. Rev. Biochem. 46, 385–413.
Thampy, K.G., and Wakil, S.J. (1985)J. Biol. Chem. 260, 6318–6323.
Goodson, J., Pope, T.S., and Allred, J.B. (1984)Biochem. Biophys. Res. Commun. 122, 694–699.
Tipper, J.P., and Witters, L.A. (1982)Biochim. Biophys. Acta 715, 162–169.
Tanabe, T., Wada, K., Okazaki, T., and Numa, S. (1975)Eur. J. Biochem. 57, 15–24.
Bai, D.H., Pape, M.E., Casillas, F., Luo, X.C., Dixon, J.E., and Kim, K. (1986)J. Biol. Chem. 261, 12395–12399.
Hardie, D.G., and Cohen, P. (1978)FEBS Lett. 91, 1–7.
Hardie, D.G., and Cohen, P. (1978)Eur. J. Biochem. 92, 25–34.
Beaty, N.B., and Lane, M.B. (1982)J. Biol. Chem. 257 (2), 924–929.
Rainwater, D.L., and Kolattukudy, P.E. (1982)Arch. Biochem. Biophys. 213 (2), 372–383.
Sinhuber, R.O., Hendricks, J.D., Wales, J.H., and Putnam, G.B. (1977)Ann. NY Acad. Sci. 298, 389–408.
Bailey, G.S., Hendricks, J.D., Nixon, J.E., and Pawlowski, N.E. (1984)Drug Metab. Rev. 15 (4), 725–750.
Hendricks, J.D. (1981) inPhyletic Approaches to Cancer (Dawe, C.J., et al., eds.), pp. 227–240, Japan Sci. Soc. Press, Tokyo.
Shelton, D.W., Hendricks, J.D., and Bailey, G.S. (1984)Toxicol. Lett. 22, 27–31.
Grieco, M.P., Hendricks, J.D., Scanlan, R.A., Sinnhuber, R.O., and Pierce, D.A. (1978)J. Natl. Cancer Inst. 60 (5), 1127–1131.
Hendricks, J.D., Sinnhuber, R.O., Loveland, N.E., Pawlowski, N.E., and Nixon, J.E. (1980)Science 208, 309–311.
Schoenhard, G.L., Hendricks, J.D., Nixon, J.E., Lee, D.J., Wales, J.H., Sinnhuber, R.O., and Pawlowski, N.E. (1981)Cancer Res. 41, 1011–1014.
Hendricks, J.D., Sinnhuber, R.O., Nixon, J.E., Wales, J.H., Masri, M.S., and Hsieh, D.P.H. (1980)J. Natl. Cancer Inst. 64 (3), 523–527.
Greene, D.H.S., and Selivonchick, D.P. (1987)Prog. Lipid. Res. 26, 53–85.
Perdew, G.H., Schaup, H.W., Becker, M.M., Williams, J.L., and Selivonchick, D.P. (1986)Biochim. Biophys. Acta 877, 9–19.
Allred, J.B., Lopez, C.R., Pope, T.S., and Goodson, J. (1985)Biochem. Biophys. Res. Commun. 129, 453–460.
Witters, L.A., Friedman, S.A., and Bacon, G.W. (1981)Proc. Natl. Acad. Sci. USA 78, 3639–3943.
Bradford, M. (1976)Anal. Biochem. 72, 248–254.
Allred, J.B., and Roehrig, K.L. (1978)J. Biol. Chem. 253, 4826–4829.
Manning, R., Dils, R., and Mayer, J. (1976)Biochem. J. 153, 463–468.
Hames, B.D. (1981) inGel Electrophoresis of Proteins—a Practical Approach (Hames, B.D., and Rickwood, D., eds.) pp. 26–27.
Bijleveld, C., and Geelen, M.J.H. (1987)Biochim. Biophys. Acta 918, 274–283.
Iritani, N., Ikeda, Y., Fukuda, H., and Katsurda, A. (1984)Lipids 19, 828–835.
Warman, A.W. III, and Bottino, N.R. (1978)Comp. Biochem. Physiol. B 59 (2), 153–162.
Hall, F.L., and Hazel, J.R. (1979)Fed. Proc. Fed. Am. Soc. Exp. Biol. 38 (3) Part 1, 1149.
Author information
Authors and Affiliations
About this article
Cite this article
McKim, J.M., Schaup, H.W., Marien, K. et al. Isolation and identification of acetyl-CoA carboxylase from rainbow trout (Salmo gairdneri) liver. Lipids 24, 187–192 (1989). https://doi.org/10.1007/BF02535233
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02535233