Abstract
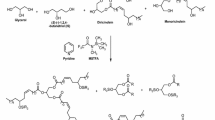
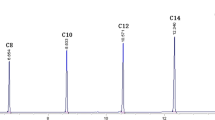
The separation conditions for hydrolysates of triglycerides by lipase and their quantitative determination are discussed for a thin layer chromatography-flame ionization detector system utilizing internal standards. The complete separation of glyceride hydrolysis mixtures (triolein 1,3-diolein, 1,2-diolein, 1-monoolein and oleic acid) was achieved on a 3% boric acid-impregnated Chromarod S-II by development with benzene/chloroform/acetic acid (70∶30∶2, v/v/v) (mobile phase A) or hexane/ ether/acetic acid (70∶30∶1, v/v/v) (mobile phase B). Mobile phase B had an advantage over mobile phase A in terms of free space to add internal standards for simultaneous quantitation and was employed.p-Hydroxybenzoic acid andp-carboethoxy benzyl alcohol, which appeared between 1,2-diolein and 1-moloolein, were adopted as the internal standards. The calibration curves relating internal standards to each glyceride were all approximated by the equations Y=aXb giving high correlations. The method was applied to hydrolysis of triolein by pancreatic lipase.
Similar content being viewed by others
Abbreviations
- TO:
-
Triolein
- DO:
-
diolein
- MO:
-
monoolein
- OA:
-
oleic acid
- HB:
-
p-hydroxybenzoic acid
- CEB:
-
p-carboethoxy benzyl alcohol
- PVA:
-
polyvinyl alcohol
References
Scott, H. (1972) in Detergency (Cutler, W.G., and Davis, R. C. eds), Vol. 5, p. 120, Marcel Dekker, New York.
Kotani, T., Fujii, T., and Okuyama, H. (1979) Yukagaku 28, 914–918.
Andree, H., Müller, C.W.R., and Schmid, R.D. (1980) J. Appl. Biochem. 2, 218–223.
Tatara, T., Fujii, T., and Minagawa, M. (1982) Presentation at 34th Annual Meeting of Jph. Soc. Home Econ., 138.
Fiore, J.V., and Nord, F.F. (1949) Arch. Biol. Chem. 23, 473–479.
Courville, D.A., and Legington, W. (1951) J. Biol. Chem. 190, 575–581.
Okumura, S., Iwai, M., and Tsujisaka, Y. (1976) Agri. Biol. Chem. 40, 655.
Benzonana, G., and Esposito, S. (1971) Biochim. Biophys. Acta 231, 15–22.
Tanaka, M., Itoh, T., and Kaneko, H. (1976) Yukagaku 25, 263–265.
Bradley, D.M., Rickards, C.R., and Thomas, N.S.T. (1979) Clin. Chim. Acta 92, 293–302.
Kramer, J.K.G., Fouchard, R.C., and Farnworth, E.R. (1980) J. Chromatogr. 198, 279–285.
Tanaka, M., Itoh, T., and Kaneko, H. (1981) Lipids 15, 872–875.
Farnworth, E.R., Thompson, B.K., and Kramer, J.K.G. (1982) J. Chromatogr. 240, 463–474.
Lowenstein, J., ed. (1981) in Methods in Enzymolology, Vol. 72(D), pp. 205–252, Academic Press, New York.
Yoshizuka, N., Okamoto, K. and Takase, Y. (1981) Kohsho Zasshi 5, 33–39.
Daubert, B.F., Friche, H.H., and Longencker, H.E. (1943) J. Am. Chem. Soc. 65, 2142–2143.
Yamada, K., Ohta, Y., and Machida, H. (1962) Nogei Kagaku Zasshi 36, 860–864.
Okumura, S., Iwai, M., and Tsujisaka, Y. (1981) Agric. Biol. Chem. 45, 185–189.
Author information
Authors and Affiliations
Additional information
Part of this investigation was reported at the annual meeting of JOCS in Tokyo, November 1982.
About this article
Cite this article
Tatara, T., Fujii, T., Kawase, T. et al. Quantitative determination of Tri-, Di-,monooleins and free oleic acid by the thin layer chromatography-flame lonization detector system using internal standards and boric acid impregnated chromarod. Lipids 18, 732–736 (1983). https://doi.org/10.1007/BF02534541
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02534541