Abstract
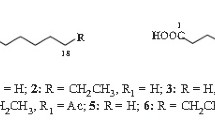
Methyl esters of 8 16-carbon acids which occur in cutin of plants have been synthesized from methyl 16,17-dihydroxy-7-oxo- and 16,17-dihydroxy-9-oxoheptadecanoates. 16-Hydroxy-7-oxo- and 16-hydroxy-9-oxohexadecanoates were prepared by lead tetraacetate cleavage followed by sodium triacetoxyborohydride reduction; 16-hydroxy-8-oxo- and 16-hydroxy-10-oxohexadecanoates were prepared by successive ketalization, ester reduction, diol cleavage, acylation, oxidation and methanolysis. Dihydroxy esters were prepared by reduction of oxo esters with sodium borohydride. Mass spectra of TMS ethers of the esters, of pyrrolides and of TMS esters of pyrrolidides have been compared. Mass spectra of mixtures of hydroxyoxo derivatives and of dihydroxy derivatives show that correction factors are required for quantitative analysis, particularly when 7-substituted isomers are present.
Similar content being viewed by others
References
Martin, J.T., and B.E. Juniper, “The Cuticles of Plants,” Edward Arnold Ltd., London, 1970.
Kolattukudy, P.E., and T.J. Walton, in “Progress in the Chemistry of Fats and Other Lipids,” Vol. 13, edited by R.T. Holman, Pergamon Press, New York, 1973, p. 121.
Matic, M., Biochem. J. 63:168 (1956).
Meakins, G.D., and R. Swindells, J. Chem. Soc. 1044 (1959).
Holloway, P.J., and A.H.B. Deas, Phytochemistry 10:2781 (1971).
Walton, T.J., and P.E. Kolattukudy, Biochemistry 11:1885 (1972).
Holloway, P.J., A.H.B. Deas and A.M. Kabaara, Phytochemistry 11:1443 (1972).
Deas, A.H.B., E.A. Baker and P.J. Holloway, Phytochemistry 13:1901 (1974).
Sisido, K., and M. Kawanisi, J. Org. Chem. 27: 3722 (1962).
Tulloch, A.P., Chem. Phys. Lipids 18:1 (1977).
Bowman, R.E., and W.D. Fordham, J. Chem. Soc. 3945 (1952).
Tulloch, A.P., Lipids 12:92 (1977).
Gribble, G.W., and D.C. Ferguson, J. Chem. Soc. Chem. Commun. 535 (1975).
Kennedy, J., A. Lewis, N.J. McCorkindale and R.A. Raphael, J. Chem. Soc. 4945 (1961).
Dauben, H.J., B. Löken and H.J. Ringold, J. Am. Chem. Soc. 76:1359 (1954).
Al Neirabeyek, M., J.-C. Ziegler and B. Gross, Synthesis 811 (1976).
Chatterjea, J.N., S.C. Sengupta, G.S. Misra and S.C. Agarwal, Indian. J. Chem. 14B:719 (1976).
Espelie, K.E., and P.E. Kolattukudy, Lipids 13:832 (1978).
Eglinton, G., and D.H. Hunneman, Phytochemistry 7:313 (1968).
Baker, E.A., and P.J. Holloway, Phytochemistry 9:1557 (1970).
Kollattukudy, P.E., and T.J. Walton, Biochemistry 11:1897 (1972).
Eglinton, G., D.H. Hunneman and A. McCormick, Org. Mass Spectrom. 1:593 (1968).
Ryhage, R., and E. Stenhagen, Ark. Kemi 15:545 (1960).
Richter, W.J., and A.L. Burlingame, J. Chem. Soc. Chem. Commun. 1158 (1968).
Valicenti, A.J., A.P. Tulloch, W.H. Heimermann and R.T. Holman, Lipids, in press.
Tulloch, A.P., Org. Mag. Reson. 11:109 (1978).
Tulloch, A.P., J.F.T. Spencer and P.A.J. Gorin, Can. J. Chem. 40:1326 (1962).
Brooks, L.A., and H.R. Snyder, in “Organic Syntheses,” Coll. Vol. 3, edited by E.C. Horning, J. Wiley, New York, 1955, p. 698.
Crossland, R.K., and K.L. Servis, J. Org. Chem. 35:3195 (1970).
Spener, F., and H.K. Mangold, Chem. Phys. Lipids 11:215 (1973).
Gaubert, P., R.P. Linstead and H.N. Rydon, J. Chem. Soc. 1971 (1937).
Author information
Authors and Affiliations
About this article
Cite this article
Tulloch, A.P. Cutin acids: Synthesis and mass spectrometry of methyl 16-hydroxy-7-oxo-, 16-hydroxy-8-oxo-, 16-hydroxy-9-oxo-, 16-hydroxy-10-oxo- and 7,16-, 8,16-, 9,16- and 10,16-dihydroxyhexadecanoates. Lipids 15, 881–888 (1980). https://doi.org/10.1007/BF02534409
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02534409