Abstract
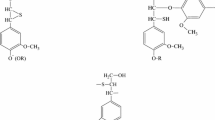
The early stages of the autoxidation of methyl hydnocarpate, chaulmoograte and gorlate in air have been examined at 40, 60 and 80 C, and the initial products have been compared by several methods with those derived from methyl oleate and linoleate autoxidized at 60 C. To supplement information about oxygen absorption and peroxide development in relation to time, other information about the early products, and some information about the reduced products, have been obtained by ultraviolet (UV) and infrared (IR) spectrophotometry, and by thin layer chromatography (TLC). The kinetic and other data presented in this study strongly support the conclusion that the methyl esters of cyclopentenyl fatty acids yield initial autoxidation products that, although they are primarily peroxides, differ in some ways (as expected) in the kinetics of their formation and their chemical nature, compared to those of oleate and linoleate. Nevertheless, all the data obtained strongly support the surmise that the peroxides are formed autocatalytically by a chain mechanism, and that secondary products not derived from peroxide decomposition, are formed pari passu in lesser, but increasing amounts with increasing temperature, probably from free radical intermediates. The autoxidation of esters of cyclopentenyl fatty acids has potential importance in several ways, 3 of which are mentioned briefly.
Similar content being viewed by others
References
Schlossberger, H., in “Handbuch der Pharmakologie, Ergänzungswerk,” Vol. 5, edited by W. Heubner, J. Schüller, Springer-Verlag, Berlin, 1938, pp. 1–141.
Jacobson, P.L., and L. Levy, Proc. West. Pharmacol. Soc. 15:44 (1972).
Mangold, H.K., and F. Spener, in “Lipids and Lipid Polymers in Higher Plants,” edited by M. Tevini and H.K. Lichtenthaler, Springer-Verlag, Berlin, 1977, p. 85.
Spener, F., and H.K. Mangold, Biochemistry 13:3342 (1974).
Hanus, J.Z., Untersuch. Nahr. Genussm. 4:913 (1901).
Luddy, F.E., R.A. Barford, S.F. Herb, and P. Magidman, J. Am. Oil Chem. Soc. (1968).
Bandi, Z.L., and H.K. Mangold, Sep. Sci. 4:83 (1969).
Zeman, I., and J. Pokorny, J. Chromatogr 10:15 (1963).
Shukla, V.K.S., E.M. Abdel-Moety, E. Larsen, and H. Egsgard, Chem. Phys. Lipids 23:285 (1979).
Umbreit, W.W., R.H. Burris, and J.F. Stauffer, “Manometric Techniques,” 4th edition, U.S.A., 1964.
Pardun, H., in “Analyse der Nahrungsfett,” Band 16, Verlag Paul Parey, Berlin, 1976, p. 34.
Privett, O.S., W.O. Lundberg, and C. Nickell, J. Am. Oil Chem. Soc. 30:17 (1953).
Bateman, L., H. Hughes, and A.L. Morris, Discuss. Faraday Soc. 14:190 (1953).
Lundberg, W.O., and J.R. Chipault, J. Am. Chem. Soc. 69:835 (1947).
Lundberg, W.O., in “Autoxidation and Antioxidants,” Vol. II, Wiley, New York and London, 1962, pp. 451–476.
Paget, H., J. Chem. Soc. 955–960 (1937).
Samuelsson, B., J. Am. Chem. Soc. 87:3011 (1965).
Author information
Authors and Affiliations
About this article
Cite this article
Abdel-Moety, E.M., Lundberg, W.O. Autoxidation of methyl esters of cyclopentenyl fatty acids. Lipids 15, 298–305 (1980). https://doi.org/10.1007/BF02533544
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02533544