Abstract
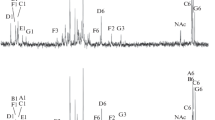
Three new 10-hydroxy fatty acids, all optically active, have been prepared by the anaerobic microbiological hydration of acis-9 double bond. Substrates that formed these new hydroxy fatty acids are linoleic, linolenic, and ricinoleic acids. The hydroxyl group has the D configuration and the methyl esters are levorotatory. Infrared, mass spectral, specific rotation and ultraviolet data on these compounds were determined. There was no migration of the unreated double bonds at C12 and C15 in linoleic or linolenic acids. The presence of a double bond in the 10-hydroxy fatty acids significantly increased the optical rotation of the methyl esters. The hydratase enzyme showed unusual specificity among Δ9 unsaturated acids. While it hydrates methylene interrupted and hydroxy unsaturated acids, it failed to hydrate either 9-decenoic, 12,13-epoxy- or 12-keto-cis-9-octadecenoic acids or sterculic acid.
Similar content being viewed by others
References
Wallen, L.L., R.G. Benedict and R.W. Jackson, Arch. Biochem. Biophys. 99:249–253 (1962).
Davis, E.N., L.L. Wallen, J.C. Goodwin, W.K. Rohwedder and R.A. Rhodes, Lipids 4:356–362 (1969).
Gunstone, F.D., J. Chem. Soc.: 1274–1278 (1952).
Wilson, T.L., C.R. Smith, Jr. and K.L. Mikolajczak, JAOCS 38:696–699 (1961).
Nichols, J., and E. Schipper, J. Amer. Chem. Soc. 80:5705–5710, 5711–5713 (1958).
Ryhage, R., and E. Stenhagen, Ark. Kemi 15:545–574 (1960).
Wu, Y.V., and J.E. Cluskey, Arch. Biochem. Biophys. 112:32–36 (1965).
Applewhite, T.H., R.G. Binder and W. Gaffield, J. Org. Chem. 32:1173–1178 (1967).
Schroepfer, G.J., Jr., and K. Bloch, J. Biol. Chem. 240:54–63 (1965).
Tulloch, A.P., and J.F.T. Spencer, Can. J. Chem. 42:830–835 (1964).
von Rudloff, E., Can. J. Chem. 34:1413–1418 (1956).
Christie, N.W., and R.T. Holman, Chem. Phys. Lipids 1:407–423 (1967).
Serck-Hanssen, K., Chem. Ind. (London) 1554 (1958).
Mizugaki, M., M. Yonaha, M. Uchiyama and S. Okui, J. Biochem. (Tokyo) 63:390–394 (1968).
Schroepfer, G.J., Jr., J. Amer. Chem. Soc. 87:1411–1412 (1965).
Author information
Authors and Affiliations
Additional information
No. Marketing and Nutrition Res. Div., ARS, USDA.
About this article
Cite this article
Wallen, L.L., Davis, E.N., Wu, Y.V. et al. Stereospecific hydration of unsaturated fatty acids by bacteria. Lipids 6, 745–750 (1971). https://doi.org/10.1007/BF02531301
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02531301