Abstract
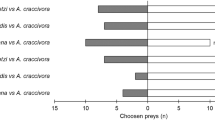
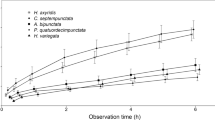
We examined food utilization in a community of aphidophagous hoverfly larvae (Diptera: Syrphidae and Chamaemyiidae) in open lands in an urban habitat in central Japan for 3 years. The community consisted of 17 hoverfly species feeding on 20 aphid species occurring on 14 species of dominant herbaceous plants. In terms of larval prey preference, the dominant eight species of hoverfly were categorized into three groups: a polyphagous ‘generalist’ group consisting of four species,Episyrphus balteatus, Betasyrphus serarius, Syrphus vitripennis andSphaerophoria sp.; an oligophagous ‘specialist’ group consisting of three species,Metasyrphus hakiensis, Dideoides latus andParagus hemorrhous; andLeucopis puncticornis, which showed a preference for two aphid species on the plantTorilis scabra. The prey aphids of the second group have behavioral or morphological defense mechanisms that are effective for preventing attacks by generalist hoverflies; two prey aphids are aggressive toward generalist predators and the others are protected by ant-attendance. The specialist hoverflies seem to be adapted to overcome these defense mechanisms. The prey ranges overlapped little between the generalist and the specialist groups, while those within the generalist group overlapped greatly.
Similar content being viewed by others
References
Aoki S. (1984) First instar larvae of the sugar-cane woolly aphid,Ceratovacuna lanigera (Homoptera, Pemphigidae), attack its predators.Kontyû 52: 458–460.
Arakaki N. (1989) Alarm pheromone eliciting attack and escape responses in the sugar woolly aphid,Ceratovacuna lanigera (Homoptera, Pemphigidae).Journal of Ethology 7: 83–90.
Arakaki N. (1990) Colony defense by the first nymphs and two functions of alarm pheromone in the sugar cane woolly aphid,Ceratovacuna lanigera.Insectarium 27: 76–81 (in Japanese).
Arakaki N. (1992) Predators of the sugar cane woolly aphid,Ceratovacuna lanigera (Homoptera: Aphididae) in Okinawa and predator avoidance of defensive attack by the aphid.Applied Entomology and Zoology 27: 159–161.
Bovbjerg R. V. (1970) Ecological isolation and competitive exclusion in two crayfish (Orconectes vivilis andOrconectes immunis).Ecology 51: 225–236.
Caswell H. (1976) Community structure; a neutral model analysis.Ecological Monograph 46: 327–354.
Connell J. H. (1983) On the prevalence and relative importance of interspecific competition: Evidence from field experiment.American Naturalist 122: 661–696.
Diamond J. M. (1973) Distributional ecology of New Guinea birds.Science 179: 759–769.
Gilbert F. S. (1985a) Morphometric patterns in hoverflies (Diptera, Syrphidae).Proceedings of the Royal Society of London B 224: 79–90.
Gilbert F. S. (1985b) Ecomorphological relationships in hoverflies (Diptera, Syrphidae).Proceedings of the Royal Society of London B 224: 91–105.
Gilbert F. S., Harding E. F., Line J. M. &Perry I. (1985) Morphological approaches to community structure in hoverflies (Diptera, Syrphidae).Proceedings of the Royal Society of London B 224: 115–130.
Gilbert F. S. &Owen J. (1985) Diurnal activity patterns in hoverflies (Diptera: Syrphidae).Ecological Entomology 10: 385–392.
Gilbert F. S. &Owen J. (1990) Size, shape, competition, and community structure in hoverflies (Diptera; Syrphidae).Journal of Animal Ecology 59: 21–39.
Hespenhide H. A. (1973) Ecological inferences from morphological data.Annual Review of Ecology and Systematics 4: 213–229.
Itioka T. &Inoue T. (1996a) The consequences of ant-attendance to the biological control of the red wax scale insectCeroplastes rubens byAnicetus beneficus.Journal of Applied Ecology 33: 609–618.
Itioka T. &Inoue T. (1996b) Density-dependent ant attendance and its effects on the parasitism of a honeydew-producing scale insect,Ceroplastes rubens.Oecologia 106: 448–454.
Itioka T. &Inoue T. (1996c) The role of predators and attendant ants in the regulation and persistence of a population of the citrus mealybugPseudococcus citriculus in a Satsuma orange orchard.Applied Entomology and Zoology 31: 195–202.
Itô Y. (1989) The evolutionary biology of sterile soldiers in aphids.Trends in Ecology and Evolution 4: 69–73.
Kurosu U. &Aoki S. (1988) First-instar aphids produced late by the fundatrix ofCeratovacuna nekoashi (Homoptera) defend their closed gall outside.Journal of Ethology 6: 99–104.
MacArthur R. H. (1960) On the relative abundance of species.American Naturalist 94: 25–36.
Ninomiya E. (1957) On the food-habits of some aphidophagous syrphid larvae II.Japanese Journal of Applied Entomology and Zoology 1: 186–192 (in Japanese with English summary).
Ninomiya E. (1968) Predation among aphidophagous insect larvae.Annual Report of Natural Science, Faculty of Education, Nagasaki University 19: 79–84 (in Japanese with English summary).
Ôhara K. (1985) Observations on the oviposition behaviour ofMetasyrphus confrater (Diptera, Syrphidae) and the defensive behaviour of soldiers ofPseudoregma bambucicola (Homoptera, Pemphigidae).Esakia 23: 99–105.
Owen J. &Gilbert F. S. (1989) On the abundance of hoverflies (Syrphidae).Oikos 55: 183–193.
Rotheray G. E. &Gilbert F. S. (1989) The phylogeny and systematics of the tribe Syrphinae (Diptera, Syrphidae) according to larval morphology.Zoological Journal of the Linnean Society 95: 27–70.
SAS (1990)SAS/STAT Users Guide. Version 6, 4th ed. SAS Institute, Cary, NC.
Schneider F. (1969) Bionomics and physiology of aphidophagous syrphidae.Annual Review of Entomology 14: 103–124.
Schoener T. E. (1974) Resource partitioning in ecological communities.Science 185: 27–39.
Schoener T. E. (1983) Field experiments on interspecific competition.American Naturalist 122: 240–285.
Strong D. R. (1983) Natural variability and manifold mechanisms of ecological communities.American Naturalist 122: 636–660.
Tatematsu Y. (1993) Interspecific competition and community structure in aphidophagous syrphidae larvae (Diptera; Syrphidae and Chamaemyiidae). Master of Agricultural Science thesis, Nagoya University, Nagoya (in Japanese with English summary).
Völkl W. (1992) Aphids or their parasitoids: who actually benefits from ant-attendance?Journal of Animal Ecology 61: 273–281.
Völkl W. (1994) The effect of ant-attendance on the foraging behaviour of the aphid parasitoidLysiphlebus cardui.Oikos 70: 149–155.
Author information
Authors and Affiliations
About this article
Cite this article
Mizuno, M., Itioka, T., Tatematsu, Y. et al. Food utilization of aphidophagous hoverfly larvae (Diptera: Syrphidae, Chamaemyiidae) on herbaceous plants in an urban habitat. Ecol. Res. 12, 239–248 (1997). https://doi.org/10.1007/BF02529453
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02529453