Abstract
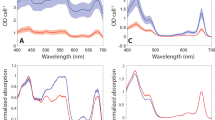
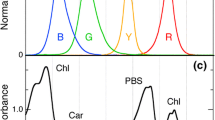
The marine cyanobacteriumPhormidium sp. strain C86 changes the phycobilisome type depending on light quality. Red-light-adapted cells contained hemidiscoidal phycobilisomes with a photosystem II:phycobilisome ratio of 2.2, while green-light-adapted cells exhibited hemiellipsoidal phycobilisomes with a photosystem II:phycobilisome ratio of 4.4, as determined by a combined analysis of freeze-fractured thylakoid membranes and ultrathin sections and by photochemical determinations of photosystems and phycobilisomes. Core complexes of phycobilisomes of red- and green-light-adapted cells were isolated by affinity chromatography and were subsequently separated into two allophycocyanin-containing fractions. The high-molecular-weight fraction, with a sedimentation coefficient of 24 S and a calculated mol. wt. of 860,000, contained complexes of the quaternary structure (α AP9 β AP8 β19.5AP)2·(LCM)2 and tricylindrical shape, previously designated APCM. This fraction was similar in size in red- and green-light-adapted cells; however, differences were detected in the low-molecular-weight allophycocyanin fraction containing the “trimeric” complexes with a sedimentation coefficient of 6 S. As shown by comparison of spectral and stoichiometric data of intact phycobilisomes and isolated core complexes, the amount of the αAPB-containing core complex (α AP2 αAPBβ AP3 ·L 10C ) was greater in core fractions of green-light phycobilisomes, whereas the amount of the core complexes (α AP3 β AP3 ·L 10C ) designated AP·L 10C , was higher in cores of red-light phycobilisomes.Phormidium sp. is the first organism examined that exhibits a new type of complementary chromatic adaptation by altering the composition of the phycobilisome core and the number and composition of peripheral rods and by changing the ratio of photosystem II to phycobilisomes. A model summarizing the structural consequences of the results is presented.
Similar content being viewed by others
Abbreviations
- AP :
-
“Trimeric” allophycocyanin complex of the structure (α AP3 β AP3 )
- APB :
-
“Trimeric” αAPB containing allophycocyanin complex of the structure (α AP2 αAPBβ AP3 )
- AP CM :
-
Allophycocyanin core membrane complex of the structure (α AP9 β AP8 β19.5AP)
- AP·L 10C :
-
“Trimeric” allophycocyanin complex of the structure (α AP3 β AP3 )·L 10C
- Chl :
-
Chlorophyll
- EF :
-
Exoplasmic fracture face
- GL :
-
Green light
- L C :
-
Phycobilisome core linker polypeptide
- L CM :
-
Phycobilisome core-membrane linker polypeptide
- P700 :
-
Reaction center of photosystem I
- PBS :
-
Phycobilisome
- PC :
-
Phycocyanin
- PE :
-
Phycoerythrin
- PF :
-
Protoplasmic fracture face
- PMSF :
-
Phenylmethanesulfonylfluoride
- PS I :
-
Photosystem I
- PS II :
-
Photosystem II
- RL :
-
Red light
- S :
-
Sedimentation coefficient
References
Anderson LK, Eiserling FA (1986) Asymmetrical core structure in phycobilisomes of the cyanobacteriumSynechocystis 6701. J Mol Biol 191:441–451
Bennett A, Bogorad L (1973) Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol 58:419–435
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem 72:248–254
Bryant DA (1988) Genetic analysis of phycobilisome biosynthesis, assembly, structure, and function in the cyanobacteriumSynechococcus sp. PCC 7002. In: Stevens SE Jr, Bryant DA (eds) Light-energy transduction in photosynthesis: higher plant and bacterial models, American Society of Photobiologists, Rockville, pp 62–90
Bryant DA (1991) Cyanobacterial phycobilisomes: progress toward complete structural and functional analysis via molecular genetics. In: Bogorad L, Vasil IK (eds) Cell culture and somatic cell genetics of plants, vol 7B. Academic Press, New York, pp 257–300
Bryant DA, Guglielmi G, Tandeau de Marsac N, Castets AM, Cohen-Bazire G (1979) The structure of cyanobacterial phycobilisomes: a model. Arch Microbiol 123:113–127
Capuano V, Braux AS, Tandeau de Marsac N, Houmard J (1991) The “anchor polypeptide” of cyanobacterial phycobilisomes. Molecular characterization of theSynechococcus sp. PCC 6301apcE gene. J Biol Chem 266:7239–7247
Clement-Metral JD, Gantt E, Redlinger T (1985) A photosystem II-phycobilisome preparation from the red algaePorphyridium cruentum: oxygen evolution, ultrastructure and polypeptide resolution. Arch Biochem Biophys 238:10–17
Cunningham FX, Dennenberg RJ, Jursinic PA, Gantt E (1990) Growth under red light enhances photosystem II relative to photosystem I and phycobilisomes in the red algaPorphyridium cruentum Plant Physiol 93:888–895
Gantt E (1981) Phycobilisomes. Annu Rev Plant Physiol 32:327–347
Giddings TH, Wasmann C, Staehelin LA (1983) Structure of the thylakoids and envelope membranes of the cyanelles ofCyanophora paradoxa Plant Physiol 71:409–419
Gindt YM, Zhou J, Bryant DA, Glazer AN, Sauer K (1992) Core mutations ofSynechococcus sp. PCC 7002 phycobilisomes: a spectroscopic study. J Photochem Photobiol B 15:75–89
Glazer AN (1985) Light harvesting by phycobilisomes. Annu Rev Biophys Biophys Chem 14:47–77
Glazer AN, Chan C, Yeh SW, Clark JH (1985) Kinetics of energy flow in the phycobilisome core. Science 230:1051–1053
Grossman AR, Lemaux PG, Conley PB, Burns BU, Anderson LK (1988) Characterization of phycobiliprotein genes inFremyella diplosiphon and their regulated expression during complementary chromatic adaptation. Photosynthesis Res 17:23–56
Hiyama T, Ke B (1972) Difference spectra and extinction coefficients of P700. Biochim Biophys Acta 267:160–171
Holzwarth AR, Bittersmann E, Reuter W, Wehrmeyer W (1990) Studies on chromophore coupling in isolated phycobiliproteins. 3. Picosecond excited state kinetics and time-resolved fluorescence spectra of different allophycocyanins fromMastigocladus laminosus. Biophys J 57:133–145
Isono T, Katoh T (1983) Subparticles ofAnabaena phycobilisomes I. Reconstitution of allophycocyanin cores and entire phycobilisomes. Plant Cell Physiol 24:357–368
Kirk JTO (1968) Studies on the dependence of chlorophyll synthesis on protein synthesis inEuglena gracilis, together with a nomogram for determination of chlorophyll concentration. Planta 78:200–207
Kume N, Isono T, Katoh T (1982) Stability of cyanobacterial phycobilisomes in reference to their concentration. Photobiochem Photobiophys 4:25–37
Kursar TA, Alberte RS (1983) Photosynthetic unit organization in a red alga. Plant Physiol 72:409–414
Lange W, Wilhelm C, Wehrmeyer W, Mörschel E (1990) The supramolecular structure of photosystem II-phycobilisome-complexes ofPorphyridium cruentum. Bot Acta 103:250–257
Lichtlé C, Thomas JC (1976) Étude ultrastructurale des thylakoides des algues à phycobiliproteines, comparison des résultats obtenus par fixation classique et cryodécapage. Phycologia 15:393–404
Lundell DJ, Glazer AN (1981) Allophycocyanin B. A common subunit inSynechococcus allophycocyanin B (λmax 670) and allophycocyanin (λmax 650). J Biol Chem 256:12600–12606
Lundell DJ, Glazer AN (1983a) Molecular architecture of a light-harvesting antenna. Core substructure inSynechococcus 6301 phycobilisomes: two new allophycocyanin and allophycocyanin B complexes. J Biol Chem 258:902–908
Lundell DJ, Glazer AN (1983b) Molecular architecture of a light-harvesting antenna. Quaternary interactions in theSynechococcus 6301 phycobilisome core as revealed by partial digestion and circular dichroism studies. J Biol Chem 258:8708–8713
Manodori A, Melis A (1984) Photochemical apparatus organization inAnacystis nidulans (Cyanophyceae). Effect of CO2 concentration during cell growth. Plant Physiol 74:67–71
Marsho TV, Kok B (1971) Detection and isolation of P700. Methods Enzymol 23:515–522
Maxson P, Sauer K, Zhou J, Bryant DA, Glazer AN (1989) Spectroscopic studies of cyanobacterial phycobilisomes lacking core polypeptides. Biochim Biophys Acta 977:40–51
Mimuro M, Lipschultz C, Gantt E (1986) Energy flow in the phycobilisome core ofNostoc sp. (MAC): two independent terminal pigments. Biochim Biophys Acta 852:126–132
Mörschel E, Schatz GH (1987) Correlation of photosystem-II complexes with exoplasmic freeze-fracture particles of thylakoids of the cyanobacteriumSynechococcus sp. Planta 172: 145–154
Mörschel E, Koller KP, Wehrmeyer W, Schneider H (1977) Biliprotein assembly in the disc-shaped phycobilisomes ofRhodella violacea. 1. Electron microscopy of phycobilisomes in situ and analysis of their architecture after isolation and negative staining. Cytobiology 16:118–129
Myers J, Graham JR (1983) On the ratio of photosynthetic reaction centers RC2/RC1 inChlorella. Plant Physiol 71:440–442
Ohki K, Fujita Y (1992) Photoregulation of the phycobilisome structure during complementary chromatic adaptation in the marine cyanobacteriumPhormidium sp. C86. J Phycol 28:803–808
Pinter J, Provasoli L (1958) Artificial cultivation of a red-pigmented marine blue-green alga,Phormidium persicinum. J Gen Microbiol 18:190–197
Redecker D, Wehrmeyer W, Reuter W (1993) Core substructure of the hemiellipsoidal phycobilisome from the red algaPorphyridium cruentum. Eur J Cell Biol 62:442–450
Reuter W, Nickel-Reuter C (1993) Molecular assembly of the phycobilisomes from the cyanobacteriumMastigocladus laminosus. J Photochem Photobiol B 18:51–66
Reuter W, Wehrmeyer W (1988) Core substructure inMastigocladus laminosus phycobilisomes. 1. Microheterogeneity in two of three allophycocyanin core complexes. Arch Microbiol 150:534–540
Reuter W, Wehrmeyer W (1990) Core substructure inMastigocladus laminosus phycobilisomes. 2. The central part of the tricylindrical core-APCM-contains the “anchor” polypeptide and no allophycocyanin B. Arch Microbiol 153:111–117
Reuter W, Nickel C, Wehrmeyer W (1990) Isolation of allophycocyanin B fromRhodella violacea results in a model of the core from hemidiscoidal phycobilisomes of rhodophyceae. FEBS Lett 273:155–158
Tandeau de Marsac N (1977) Occurrence and nature of chromatic adaptation in cyanobacteria. J Bacteriol 130:82–91
Tandeau de Marsac N (1983) Phycobilisomes and complementary chromatic adaptation in cyanobacteria. Bull Inst Pasteur 81:201–254
Tandeau de Marsac N, Houmard J (1993) Adaptation of cyanobacteria to environmental stimuli: new steps towards molecular mechanisms. FEMS Microbiol Rev 104:119–190
Tandeau de Marsac N, Mazel D, Damerval T, Guglielmi G Capuano V, Houmard J (1988) Photoregulation of gene expression in the filamentous cyanobacteriumCalothrix sp. PCC 7601: light-harvesting complexes and cell differentiation. Photosynthesis Res 18:99–132
Wehrmeyer W, Zimmermann C, Ohki K, Fujita Y (1988) On a new type of cyanobacterial phycobilisome exemplified inPhormidium persicinum. Eur J Cell Biol 46:539–546
Wehrmeyer W, Mörschel E, Vogel K (1993) Core substructure in phycobilisomes of red algae. 2. The central part of the tricylindrical core-APCM-a constituent of hemidiscoidal phycobilisomes ofRhodella violacea. Eur J Cell Biol 60:203–209
Westermann M (1992) Biochemische und ultrastrukturelle Charakterisierung der hemidiscoidalen und hemiellipsoidalen Phycobilisomentypen vonPhormidium sp. (Cyanobacteria). PhD thesis. Philipps-Universität Marburg
Westermann M, Reuter W, Schimek C, Wehrmeyer W (1993) Presence of both hemidiscoidal and hemiellipsoidal phycobilisomes in aPhormidium species (Cyanobacteria). Z Naturforsch [C] 48:28–34
Yamanaka G, Lundell DJ, Glazer AN (1982) Molecular architecture of a light-harvesting antenna. Isolation and characterization of phycobilisome subassembly particles. J Biol Chem 257: 4077–4086
Zhao J, Zhou J, Bryant DA (1992) Energy transfer process in phycobilisomes as deduced from analyses of mutants ofSynechococcus sp. PCC 7002. In: Murata N (ed) Research in photosynthesis, vol 1. Kluwers, Dordrecht Boston London, pp 25–32
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Westermann, M., Wehrmeyer, W. A new type of complementary chromatic adaptation exemplified byPhormidium sp. C86: Changes in the number of peripheral rods and in the stoichiometry of core complexes in phycobilisomes. Arch. Microbiol. 164, 132–141 (1995). https://doi.org/10.1007/BF02525319
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02525319