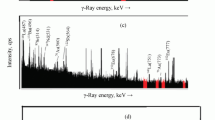
Abstract
A systematic non-destructive determination of eighteen trace elements (F, Na, Cl, Sc, Mn, Zn, Br, Sr, I, Ba, La, Ce, Sm, Eu, Tb, Yb, Th and U) in carbonate samples by thermal neutron activation analysis was developed. Three 0.2–0.5g samples were irradiated for 15 sec (in the case of determination of F), for 3 min (in the case of Na, Cl, Mn, Sr and I) and for 60 hrs (in the case of Sc, Zn, Br, Ba, La, Ce, Sm, Eu, Tb, Yb, Th and U) in the TRIGA MARK II Reactor at a thermal neutron flux of 5·1011 n·cm−2·sec−1 (15 sec and 3 min irradiation) and 1.5·1012n·cm−2·sec−1 (60 hrs irradiation), respectively. According to the half life of the nuclides formed, the activities were measured with a Ge(Li) spectrometer as follows,20F∶15 sec counting after 20–25 sec cooling,24Na,38Cl,56Mn,87mSr and128I∶600 sec couting after 30–120 min cooling,82Br,140La,153Sm,175Yb and239Np (daughter of239U)∶3000 sec counting after 1 week cooling,46Sc,65Zn,131Ba,141Ce,152Eu,160Tb and233Pa (daughter of233Th)∶5000 sec counting after 1 month cooling. The errors due to the fluctuation of the neutron flux and the counting geometry were minimized by the use of calcium determined previously with EDTA-titration as an internal standard. The interferences from24Mg(n, p)24Na and235U(n, fission) reactions were corrected by the activities produced by the reactions in unit weight of magnesium and uranium, and their concentrations in samples measured experimentally. The data of Na, Mn, Zn and Sr were compared with the results obtained by atomic absorption analysis.
Similar content being viewed by others
References
J. N. WEBER, Geochim. Cosmochim. Acta, 28 (1964) 1817.
C. O. INGAMELLS, N. H. SUHR, Geochim. Cosmochim. Acta, 31 (1967) 1347.
G. THOMPSON, D. C. BANKSTON, S. M. PASLEY, Chem. Geol., 6 (1970) 165.
A. SCHOFIELD, L. HASKIN, Geochim. Cosmochim. Acta, 28 (1964) 437.
P. BRÄTTER, P. MÖLLER, U. RÖSICK, Proc. Nato Advanced Study Institute on Activation Analysis in Geochemistry and Cosmochemistry, Scandinavian Univ. Books, Oslo, 1970, p. 461.
N. OHTA, K. TOMURA, M. OMORI, J. Chem. Soc. Japan, (1972) 1860.
N. OHTA, K. TOMURA, M. OMORI, J. Chem. Soc. Japan, (1974) 703.
N. OHTA, K. TOMURA, M. OMORI, J. Chem. Soc. Japan, (1976) 1076.
H. HIGUCHI, K. TOMURA, H. HAMAGUCHI, J. Radioanal. Chem., 5 (1970) 207.
D. F. COVELL, Anal. Chem., 31 (1959) 1785.
K. TOMURA, N. OHTA, J. Radioanal. Chem., 34 (1976) 475.
I. M. H. PAGDEN, G. J. PEARSON, J. M. BEWERS, J. Radioanal. Chem., 9 (1971) 101.
R. DAMS, F. ADAMS, Radiochim. Acta, 10 (1968) 1.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ohde, S., Ohta, N. & Tomura, K. Determination of trace elements in carbonates by instrumental neutron activation analysis. J. Radioanal. Chem. 42, 159–167 (1978). https://doi.org/10.1007/BF02520636
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02520636