Abstract
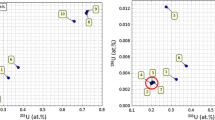
A rapid non-destructive neutron activation technique for the determination of the238U/235U ratio is described. Reagent grade uranium salts from commercial sources have a widely variable238U/235U ratio. The isotopic composition of uranium found in such salts is quite different from the natural value. This difference is largely due to the use of by-product uranium depleted in235U.
Similar content being viewed by others
References
J. W. MORGAN, J. F. LOVERING, Anal. Chim. Acta, 32 (1963) 405.
A. O. NIER, Phys. Rev., 55 (1939) 150.
P. R. FIELDS, H. DIAMOND, D. METTA, C. STEVENS, D. ROKOP, P. MORELAND, Geochim. Cosmochim. Acta, Suppl. 1, 34 (1970) 1097.
H. HAMAGUCHI, G. W. REED, A. TURKEVICH, Geochim. Cosmochim. Acta, 12 (1957) 337.
M. TATSUMOTO, J. N. ROSHOLT, Science, 167 (1970) 461.
G. T. SEABORG, Encyclopedia of the Chemical Elements C. A. HAMPEL (Ed.), Reinhold, p. 776.
A. O. NIER, Phys. Rev., 55 (1939) 153.
P. K. KURODA, J. Chem. Phys., 25 (1956) 781.
P. K. KURODA, J. Chem. Phys., 25 (1956) 1295.
R. BODU, H. BOUZIGNES, N. MORIN, J. P. PFIFFLEMANN, C. R. Acad. Sci. Paris, 275D (1972) 1731.
G. C. COWAN, H. H. ADLER, Geochim. Cosmochim. Acta, 40 (1976) 1487.
I. L. BARNES, E. L. GARNER, J. W. GRAMLICH, L. J. MOORE, T. J. MURPHY, L. A. MACHLAN, W. R. SHIELDS, M. TATSUMOTO, R. J. KNIGHT, Anal. Chem., 45 (1973) 880.
H. KÖNIG, H. WÄNKE, Z. Naturforsch., 14a (1959) 866.
J. W. MORGAN, J. F. LOVERING, Geochim. Cosmochim. Acta, 37 (1973) 1697.
R. S. CLARK, M. W. ROWE, R. GANAPATHY, P. K. KURODA, Geochim. Cosmochim Acta, 31 (1967) 1605.
U. KRÄHENBÜHL, J. W. MORGAN, R. GANAPATHY, E. AANDERS, Geochim. Cosmochim. Acta, 37 (1973) 1353.
National Bureau of Standards Special Publication 260 June 1975, 88 p.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ganapathy, R. Reagent grade uranium salts: Isotopic composition by neutron activation. J. Radioanal. Chem. 44, 199–206 (1978). https://doi.org/10.1007/BF02517690
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02517690