Abstract
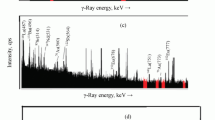
A simple method is presented for the determination of arsenic in rocks and in sediments by neutron activation. After irradiating a sample it is, without any other treatment, directly heated in condensed phosphoric acid containing sodium chloride or sodium bromide to evolve arsenic as arsenic(III) chloride or bromide. The distillate is absorbed in distilled water, in which arsenic is later precipitated in elementary form by adding hypophosphite to the solution. From arsenite, arsenic(III) oxide, arsenate and arsenic(III) sulphide, arsenic chloride can be evolved with NaCl-CPA reagent, but elementary arsenic and arsenic(V) oxide do not react with it. However, metallic arsenic is found to react with KIO3-NaCl-CPA and arsenic(V) oxide with the NaBr-CPA, both both evolving arsenic(III) chloride or bromide. Therefore, successive distillations, the first with NaCl-CPA and the second with NaBr-CPA, give a satisfactory means of differential determination of arsenic(III) and arsenate as well as arsenic(V) oxide. For the elementary arsenic a problem still now remains. The chemical recovery of carrier goes well beyond 95%.
Similar content being viewed by others
References
L. H. AHRENS, S. R. TAYLOR, Spectrochemical Analysis, 2nd ed., Addison-Wesley, 1961.
H. ONISHI, E. B. SANDELL, Geochim. Cosmochim. Acta, 7 (1955) 1:
H. ONISHI, E. B. SANDELL, Mikrochim. Acta, (1953) 34.
J. E. PORTMANN, J. P. RILEY, Anal. Chim. Acta, 31 (1964) 509.
S. TERASHIMA, Japan Analyst (Bunseki Kagaku), 23 (1974) 1331.
A. A. SMALES, D. MAPPER, K. F. FOUCHÉ, Geochim. Cosmochim. Acta, 31 (1967) 673.
W. KIESL, Modern Trends in Activation Analysis, NBS. Special Publ., No. 312, Vol. 1, U.S. Department of the Interior, 1969.
O. LANDSTRÖM, K. SAMSAHL, C. G. WENERR, Modern Trends in Activation Analysis, NBS Special Publ., No. 312, Vol. 1, U.S. Department of the Interior, 1969.
G. H. MORRISON, J.-T. GERARD, A. TRAVESI, R. L. CURRIE, S. F. PETERSON, N. M. POTTER, Anal. Chem., 41 (1969) 1633.
J. C. LAUL, D. R. CASE, M. WECHTER, F. SCHMIDT-BLECK, M. E. LIPSHUTZ, J. Radioanal. Chem., 4 (1970) 241.
K. TERADA, S. HIRAKAWA, T. KIBA (Unpublished work).
K. TERADA, T. OOBA, T. KIBA, Talanta, 22 (1975) 41.
K. TERADA, K. MATSUMOTO, T. KIBA, Bull. Chem. Soc. Japan, 48 (1975) 2567.
W. M. LATIMER, Oxidation Potentials, 2nd ed., Prentice-Hall, 1961.
P. J. OAKLEY, Quoted in A. ANDO, H. KURASAWA, T. OHMORI, E. TAKEDA, Geochem. J., 8 (1974) 175.
Author information
Authors and Affiliations
Additional information
Part of this work was performed at the Research Reactor Institute, Kyoto University.
Rights and permissions
About this article
Cite this article
Terada, K., Okuda, K. & Kiba, T. Neutron activation analysis of arsenic in rocks and sediments by direct evolution with chloride- and bromide-condensed phosphoric acid reagents. J. Radioanal. Chem. 36, 47–58 (1977). https://doi.org/10.1007/BF02516251
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02516251