Abstract
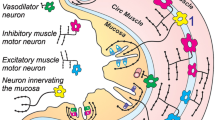
The effect of electrical stimulation of both cervical vagal nerves on mucosal mast cells in the jejunum was investigated in an in vivo animal model with rats of both sexes. Males showed a significant increase of mast cell densities after electrical stimulation (1.0 mA, 5 Hz, 5 ms, 12 min) in the lamina propria. Simultaneously, we observed a significant increase of tissue histamine levels (ANOVA:P<0.05), whereas serum levels remained unchanged. However, even though females had significantly higher levels throughout compared to males (ANOVA;P<0.05), they did not show any significant reaction to electrical stimulation. These in vivo data support morphological and in vitro data from other investigators, who hypothesized a functional interaction between mucosal mast cells and nerves. However, degranulation seems to be a poor in situ indicator for mast-cell stimulation, as mastcell densities increased in males, while the percentage of degranulated cells remained the same in all groups (about 40%). Instead, electrical stimulation of the vagal nerve seems to trigger histamine synthesis, or simply stabilization of mast cells. Interestingly, this phenomenon seems to be sex-dependent, suggesting a regulatory role for sex hormones in this scenario.
Zusammenfassung
Im Rahmen einer tierexperimentellen Studie an der Ratte wurde der Effekt einer elektrischen In-vivo-Stimulation des zervikalen N. vagus auf die Mukosamastzellen im Jejunum untersucht. Dabei zeigten männliche Tiere nach Stimulation mit 1,0 mA (5 Hz, 5 ms, 12 min) eine deutliche Zunahme der Mastzelldichte in der Lamina propria; gleichzeitig kam es zu einem signifikanten Anstieg des Gewebehistaminspiegels (ANOVA:p<0,05), während die Serumspiegel nicht signifikant anstiegen. Anders sah dies bei den weiblichen Tieren aus, die zwar im Vergleich zu den Männchen durchweg höhere Ausganswerte boten (p<0,05), bei denen jedoch die elektrische Stimulation keine signifikanten Veränderungen bewirkte. Diese Studie bestätigt in vivo die Bedeutung morphologischer und funktioneller In-vitro Daten, die eine funktionelle Interaktion zwischen Mukosamastzellen und Nerven nahelegen. Im Gegensatz zu den klassischen allergischen Reaktionen löst die elektrische Stimulation jedoch offensichtlich keine Degranulation der Mastzelle aus (ca. 40% degranulierte Mastzellen in allen Gruppen), sondern eher eine Stabilisierung oder verstärkte Histaminsynthese der Mastzellen. Zusätzlich scheinen die Phänomene der Neuroimmunmodulation teilweise von Sexualhormonen beeinflußt zu werden, wie die Geschlechtsunterschiede nahelegen.
Similar content being viewed by others
Literatur
Ader R (1992) On the clinical relevance of psychoneuroimmunology. Clin Immunol Immunopathol 64:6–9
Aldenborg F, Enerbäck L (1985) Histamine content and mast cell numbers in tissues of normal and athymic rats. Agents Actions 17:454–459
Arizono N, Matsuda S, Hattori T, Kojima Y, Maeda T, Galli SJ (1990) Anatomical variation in mast cell nerve associations in the rat small intestine, heart, lung, and skin: Similarities between neural processes and mast cells, eosinophils, or plasma cells in the jejunal lamina proprial. Lab Invest 62:626–634
Bäck N, Ahonen M, Häppölä O, Kivilaakso E, Kiviluoto T (1994) Effet of vagotomy on expression of neuropeptides and histamine in rat oxyntic mucosa. Dig Dis Sci 2:353–361
Berthoud HR, Carlson NR, Powley TL (1991) Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol 260:R200-R207
Betancur C, Neveu PJ, Vitiello S, Le Moal M (1991) Natural killer cell activity is associated with brain asymmetry in male mice. Brain Behav Immun 5:162–169
Cooke H (1986) Neurobiology of the intestinal mucosa. Gastroenterology 40:1057
Dimitriadou V, Buzzi MG, Moskowitz MA, Theoharides TC (1991) Trigeminal sensory fiber stimulation induces morphological changes reflecting secretion in rat dura mater. Neuroscience 44:97–107
Enerbäck L (1966) Mast cells in rat gastrointestinal mucosa. 2. Dye binding and metachromatic properties. Acta Pathol Microbiol Scand 66:303–313
Fewtrell CM, Foreman JC, Jordan CC, Oehme P, Renner H, Stewart JM (1982) The effects of substance P on histamine and 5-hydroxy-tryptamine release in the rat. J Physiol 330:393–411
Galli SJ (1993) New concepts about the mast cell. N Engl J Med 328:257–265
Gaytan F, Bellido C, Carrera G, Aguilar E (1990) Differentiation of mast cells during postnatal development of neonatally estrogen-treated rats. Cell Tissue Res 259:25–31
Gordon JR, Burd PR, Galli SJ (1990) Mast cells as a source of multifunctional cytokines. Immunol Today 11:458–464
Gottwald TP, Hewlett BR, Lhoták Ŝ, Stead RH (1995) Electrical stimulation of the vagus nerve modulates histamine content and mast cells in the rat jejunal mucosa. Neuroreport 7:313–317
Grossman JC, Roselle GA, Medenhall CL (1991) Sex steroid regulation of autoimmunity. J Steroid Biochem Mol Biol 40:649–659
Hirschowitz BI, Groarke J (1993) Vagal effects on acid and pepsin secretion and serum gastrin in duodenal ulcer and controls. Dig Dis Sci 38:1874–1884.
Jankoric BD (1989) Neuroimmunomodulation. Facts and dilemmas. Immunol Lett 21:101–118
Kiernan JA (1990) Degranulation of mast cells in the trachea and bronchi of the rat following stimulation of the vagus nerve. Int Arch Allergy Immunol 91:398–406
MacQueen G, Marshall J, Perdue M, Siegel S, Bienenstock J (1989) Pavlovian conditioning of rat mucosal mast cell protease II. Science 243:83–85
Marchetti B, Gallo F, Farinella Z, Romeo C, Morale MC (1996) LHRH receptors in the neuroendocrine-immune network. Biochemical bases and implications for reproductive physiopathology. Ann N Y Acad Sci 784:209–236
McDermott MR, Clark DA, Bienenstock J (1980) Evidence for a common mucosal immune system. N. Influence of estrus cycle on B immonoblast migration into genital and intestinal tissue. J Immunol 124:2436–2439
Mirski S, Befus AD, Bienenstock J (1983) Effects of sex differences and gonadal hormone alterations on the accumulation of mesenteric lymphoblasts in small intestine. Ann N Y Acad Sci 409:845–847
Ottway C.A. (1991) Neuroimmunomodulation in the intestinal mucosa. Gastroenterol Clin North Am 20:511–529
Shaff RE, Beaven MA (1979) Increased sensitivity of the enzymatic isotopic assay of histamine in plasma and serum Anal Biochem 94:425–430
Shanahan F, Anton P (1988) Neuroendocirne modulation of the immune system. Dig Dis Sci 33:41S-49S
Skofitsch G, Savitt JM, Jacobowitz DM (1985) Suggestive evidence for a functional unit between mast cells and substance P fibers in the rat diaphragm and mesentery. Histochemistry 81:5–8
Stach W (1973) Über die Nervengeflechte der Duodenalzotten: licht- und elektronenmikroskopische Untersuchungen. Acta Anat 85:216–231
Stanisz AM, Kataeva G, Bienenstock J (1994) Hormones and local immunity. Int Arch Allergy Immunol 103:217–222
Stead RH, Dixon MF, Bramwell NH, Riddell RH, Bienenstock J (1989) Mast cells are closely apposed to nerves in the human gastrointestinal mucosa. Gastroenterology 97:575–585
Stead RH, Tamioka M, Riddell RH, Bienenstock J (1988) Substance P and/or calcitonin gene-related peptide are present in subepithelial enteric nerves apposed to intestinal and mucosal mast cells. In: McDermott RP (ed) Inflammatory bowel disease. Current status and future approach. Elsvier, Amsterdam New York
Williams RM, Bienenstock J, Stead RH (1995) Mast cells: the neuroimmune connection. Chem Immunol 61:208–235
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gottwald, T., Becker, H.D., Stead, R.H. et al. Geschlechtsunterschiede Bei Der Neuromodulation von Mukosamastzellen im Rattenjejunum. Langenbecks Arch Chiv 382, 157–163 (1997). https://doi.org/10.1007/BF02498669
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02498669