Abstract
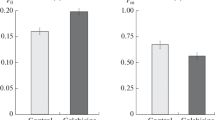
Tubulin contents in the extract from cultured carrot cells at different growth phases were investigated by measuring colchicine-binding activity. The addition of vinblastine and dithiothreitol to the reaction mixture appreciably improved the stability of both free and colchicine-bound tubulins. Colchicine-binding activity in the cell extract obtained from stationary phase was more labile than that from log phase though the extract showed higher affinity to colchicine. After purification, however, tubulin from the cells at different growth phases showed the same affinity and its colchicine-binding activity was much more stable than in crude extract. The colchicine-binding activity in the crude extract was corrected for the decay during measurement and apparent difference in the affinity so that the activity in the cells containing different kind and amount of interefering substances could be compared. The corrected amount of colchicine that binds to the 100,000×g extract was 46 pmol/105 cells at log phase. It decreased with the progression of culture age from linear to stationary phase. Combining the data with the morphological observation, it was suggested that the log phase cells contained larger free tubulin pool than the linear or stationary phase cells.
Similar content being viewed by others
Abbreviations
- DTT:
-
dithiothreitol
- IBC:
-
initial binding capacity
- IBC:
-
initial binding capacity at infinite colchicine concentration
- PM:
-
10 mM potassium phosphate, 10 mM magnesium sulfate (pH 6.9)
- TPM:
-
1 M tartrate, 10 mM potassium phosphate, 10 mM magnesium sulfate (pH 6.9)
References
Asamizu, T., N. Nakano andA. Nishi 1983. Change in non-cellulosic cell wall polysaccharide during the growth of carrot cells in suspension cultures. Planta158: 166–174.
Cyr, R.J., M.M. Bustos, M.J. Guiltinan andD.E. Fosket. 1987. Developmental modulation of tubulin protein and mRNA levels during somatic embryogenesis in cultured carrot cells. Planta171: 365–376.
DeHoff, R.T., andF.N. Rhines. 1968. Quantitative Microscopy. McGrow-Hill Publishing Co., New York (translated in Japanese, Uchida Rokakuho Publishing Co. Tokyo, 1972).
Guiltinan, M.J., D.-P. Ma, R.F. Barker, M.M. Bustos, R.J. Cyr, R. Yadegari andD.E. Fosket. 1987. The isolation, characterization and sequences of two divergent betatubulin genes from soybean (Glycine max L.). Plant Mol. Biol.10: 171–184.
Hart, J.W. andD.D. Sabnis. 1976. Colchicine-binding activity in extracts of higher plants. J. Exp. Bot.27: 1353–1360.
Hussey, P.J., C.W. Lloyd andK. Gull. 1988. Differential and developmental expression of beta-tubulin in a higher plant. J. Biol. Chem.263: 5474–5479.
—,J.A. Traas, K. Gull andC.W. Lloyd. 1987 Isolation of cytoskeletons from synchronized plant cells: the interphase microtubule array utilizes multiple tubulin isotypes. J. Cell Sci.88: 225–230.
Kato, T., M. Kakiuchi andS. Okamura. 1985. Properties of purified colchicine-binding protein from cultured carrot cell extract. J. Biochem.98: 371–377.
Ledbetter, M.C. andK.R. Porter. 1970. Introduction to the Fine Structure of Plant Cells. Springer Verlag, Berlin.
Ludwig, S.R., D.G. Oppenheimer, C.D. Silflow andD.P. Snustad. 1987. Characterization of the alpha-tubulin gene family ofArabidopsis thaliana. Proc. Natl. Acad. Sci. USA84: 5833–5837.
————. 1988. The alpha 1-tubulin gene family ofArabidopsis thaliana: Primary structure and preferential expression in flowers. Plant Mol. Biol.10: 311–321.
Morejohn, L.C., T.E. Bureau, L.P. Tocchi andD.E. Fosket. 1987. Resistance ofRosa microtubule polymerization to colchicine results from a low-affinity interaction of colchicine and tubulin. Planta170, 230–241.
Marks, M.D., J. West andD.P. Weeks 1987. The relatively large beta-tubulin gene family ofArabidopsis contains a member with an unusual transcribed 5′ noncoding sequence. Plant Mol. Biol.10: 91–104.
Murashige, T. andF. Skoog. 1962. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant.15: 473–497.
Okamura, S. 1980. Binding of colchicine to a soluble fraction of carrot cells in suspension. Planta149, 350–354.
— 1983. Effect of tartrate on the colchicine-binding activity in cultured carrot cell extract. Protoplasma118: 199–205.
— andI. Azumano. 1988. Primary structure of carboxy-terminal region of a higher plant beta-tubulin. Biochem. Int.16: 1103–1109.
—,H. Ishikawa, N. Suzuki andE. Yamada. 1977. Quantitative changes in organelles of mouse leukemia cell during cell cycling. Cell Struct. Funct.2: 229–240
—,T. Kato andA. Nishi. 1984. Lack of inhibition of carrot colchicine-binding activity by podophyllotoxin. FEBS Letters168: 278–280.
—,K. Sueki andA. Nishi. 1975. Physiological changes of carrot cells in suspension culture during growth and senescence. Physiol. Plant.33: 251–255.
Oppenheimer, D.G., N. Haas, C.D. Silflow andD.P. Snustad. 1988. The beta-tubulin gene family ofArabidopsis thaliana: Preferential accumulation of the beta-1 transcript in roots. Gene63: 87–102.
Potty, V.H. 1969. Determination of proteins in the presence of phenols and pectins. Anal. Biochem.29: 535–539.
Reaven, E.P., Y. Cheng andM.D. Miller. 1977. Quantitative analysis of tubulin and microtubule components in isolated rat hepatocytes. J. Cell Biol.75: 660–672.
Scatchard, G. 1949. The attraction of proteins for small molecules and ions. Ann. N.Y. Acad. Sci.51: 660–672.
Wick, S.M., R.W. Seagull, M. Osborn, K. Weber andB.E.S. Gunning 1981. Immunofluorescence microscopy of organized plant cells. J. Cell Biol.89: 685–690.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Okamura, S., Kakiuchi, M., Sano, A. et al. Colchicine-binding activity of carrot tubulin at different culture age. Bot Mag Tokyo 105, 503–513 (1992). https://doi.org/10.1007/BF02497663
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02497663