Abstract
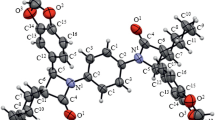
The stereochemistry of addition of Br2 toE-andZ-(R)-N-cinnamoyl-4-phenyloxazolidin-2-ones was studied. It was established that both theE-andZ-isomers give only two out of four possible diastereoisomers in the presence of Lewis acids (BPr3 or AlBr3). The absolute configurations of the diastereoisomers [(2S', 3R') and (2R', 3S') of the side chain] were established by X-ray structural analysis. The stereochemistry observed is a consequence of the stepwise bromination and the absence of bridging bromine atoms along the reaction coordinate. In the case of theZ-isomer, the diastereoselectivity of the reaction was high, whereas it is low in the case of theE-isomer. It was suggested that at the first stage of addition of Br2 at the C=C bond, the attack of the Br+ cation occurs at the α position, and the second stage of transfer of Br− occurs with the participation of boron or aluminum complexes in the intermediate state of the bromination reaction. This hypothesis as well as the results of calculations of the initial conformations of the substrates provide an explanation of the regularities observed.
Similar content being viewed by others
References
L. Duhamel, P. Angibaud, J. R. Desmurs, and J. B. Valnot,Synlet, 1991, 807.
D. A. Evans, T. C. Britton, J. A. Ellman, and R. L. Dorow,J. Am. Chem. Soc., 1990,112, 4011.
G. Li, C. Russell, M. Jarosinski, and V. J. Hruby,Tetrahedron Lett., 1993, 2565.
C. Giordano and G. Cataldi,J. Org. Chem., 1989,54, 1470.
G. Bellucci, C. Chiappe, and F. D'Andrea,Tetrahedron, Asymmetry, 1995,6, 221.
D. A. Evans, K. T. Chapman, and J. Bisaha,J. Am. Chem. Soc., 1988,110, 1238.
K. Gothelf, I. Thomsen, and K. Jorgensen,J. Am. Chem. Soc., 1996,118, 59.
R. C. Fahey and H. Schneider,J. Am. Chem. Soc., 1968,90, 4429.
V. I. Tararov, T. F. Savel'eva, Yu. N. Belokon', Yu. T. Struchkov, A. R. Pisarevskii, and N. I. Raevskii,Izv. Akad. Nauk, Ser. Khim., 1996, 640 [Russ. Chem. Bull., 1996,45, 600 (Engl. Transl.)].
D. A. Evans and J. V. Nelson,Topics Stereochem., 1982,13, 1.
E. Nicolas, K. C. Russel, and V. J. Hruby,J. Org. Chem., 1993,58, 766.
B. M. Mikhailov and Yu. N. Bubnov,Bororganicheskie soedineniya v organicheskom sinteze [Organoboron Compounds in Organic Synthesis], Nauka, Moscow, 1977 (in Russian).
J. R. Johnson, H. R. Snyder, and M. G. van Campen,J. Am. Chem. Soc., 1938,60, 115.
F. D'Angel, P. Marchetti, and V. Bertolusi,J. Org. Chem., 1995,60, 4013.
Author information
Authors and Affiliations
Additional information
Translated fromIzvestiya Akademii Nauk. Seriya Khimicheskaya, No. 5, pp. 1022–1028, May, 1997.
Rights and permissions
About this article
Cite this article
Belokoń, Y.N., Bubnov, Y.N., Churkina, T.D. et al. Diastereoselective bromination of(R)-N-cinnamoyl-4-phenyloxazolidin-2-one in the presence of Lewis acids. Russ Chem Bull 46, 982–989 (1997). https://doi.org/10.1007/BF02496131
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02496131