Abstract
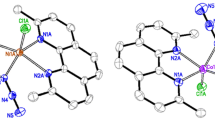
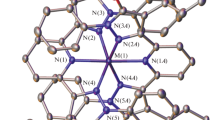
The reaction of NiCl2·6H2O with Me3CCOOH and KOH taken in a molar ratio of 1:2:2 in water afforded the nonanuclear antiferromagnetic complex Py2Ni2(Me3CCOOH)2(OOCCMe3)2(μ-OOCCMe3)2(μ-OH2), which apparently contains NiII and NiIII atoms. The complex was isolated by extraction with CH2Cl2, benzene, or hexane. The reactions of this complex with pyridine bases (pyridine (Py), 3,4-lutidine (Lut), and nicorandil (Nic)) gave the adducts L4Ni2(OOCCMe3)2(μ-OOCCMe3)2(μ-OH) (L=Py, Lut, or Nic, respectively). According to magnetic measurements, intramolecular ferromagnetic exchange interactions in these adducts are complemented by intermolecular antiferromagnetic interactions. Pyrolysis of the pyridine adduct in air or under an inert atmosphere in xylene yielded the antiferromagnetic complex Py2Ni2(Me3CCOOH)2(OOCCMe3)2(μ-OOCCMe3)2(μ-OH2), which contains NiII atoms. The structures of all the complexes synthesized were established by X-ray diffraction analysis. The electronic absorption spectra of these compounds are considered.
Similar content being viewed by others
References
I. L. Eremenko, M. A. Golubnichaya, S. E. Nefedov, I. B. Baranovskii, I. A. Ol'shnitskaya, O. G. Ellert, V. M. Novotortsev, L. T. Eremenko, and D. A. Nesterenko,Zh. Neorg. Khim., 1996,41, 2029 [Russ. J. Inorg. Chem., 1996,41 (Engl. Transl.)].
I. L. Eremenko, M. A. Golubnichaya, S. E. Nefedov, A. A. Sidorov, D. A. Nesterenko, N. P. Konovalova, L. M. Volkova, and L. T. Eremenko,Izv. Akad. Nauk, Ser. Khim., 1997, 1672 [Russ. Chem. Bull., 1997,46, (Engl. Transl.)].
A. A. Pasynskii, T. Ch. Idrisov, K. M. Suvorova, and V. T. Kalinnikov,Koord. Khim., 1976,2, 1060. [Sov. J. Coord. Chem., 1976,2 (Engl. Transl.)].
J. Catterick and P. Tornton,J. Chem. Soc., Dalton Trans., 1975, 233.
A. B. Blake and L. R. Fraser,J. Chem. Soc., Dalton Trans., 1975, 193.
A. P. Galua, G. V. Novitskii, G. A. Timko, and I. Sandu,Koord. Khim., 1994,20, 290 [Russ. J. Coord. Chem., 1994,20 (Engl. Transl.)].
N. V. Gerbeleu, G. A. Timko, O. S. Manole, Yu. T. Struchkov, A. S. Batsanov, and S. V. Grebenko,Koord. Khim., 1994,20, 357 [Russ. J. Coord. Chem., 1994,20 (Engl. Transl.)].
K. Asamaki, T. Nakamoto, S. Kawata, H. Sano, M. Katada, and K. Endo,Inorg. Chim. Acta, 1995,236, 155.
A. B. P. Lever,Studies in Physics and Theoretical Chemistry, Vol. 33: Inorganic Electronic Spectroscopy, 2nd. ed., Elsevier, Amsterdam, 1984.
C. J. Ballhausen,Introduction to Ligand Field Theory, McGraw-Hill Book Co., New York, 1962.
D. C. McClure,J. Chem. Phys., 1962,36, 2757.
Von D. Reinen, C. Friebel, and V. Propach,Z. Anorg. Allg. Chem., 1974,408, 187.
N. I. Kirilova, Yu. T. Struchkov, M. A. Porai-Koshits, A. A. Pasynskii, A. S. Antsyshkina, L. Kh. Minacheva, G. G. Sadikov, T. Ch. Idrisov, and V. T. Kalinnikov,Inorg. Chim. Acta, 1980,40, 115.
N. Hirashima, S. Nusebye, M. Kato, K. Maartmann-Moe, Y. Muto, M. Nakashima, and T. Tokaii,Acta Chem. Scand., 1990,44, 984.
M. Morooka, S. Ohba, M. Nakashima, T. Tokaii, Y. Muto, M. Kato, and O. W. Steward,Acta. Crystallogr., 1992,C48, 1888.
C. H. Kennard, E. J. O'Reilly, and G. Smith,Polyhedron, 1984,3, 689.
M. Ahngren and U. Turpeinen,Acta Crystallogr., 1982,B38, 276.
P. Chaudhuri, H. -J. Kuppers, K. Wieghardt, S. Gehring, W. Haase, B. Nuber, and J. Weiss,J. Chem. Soc., Dalton Trans., 1988, 1367.
P. W. Anderson and H. Hasegawa,Phys. Rev., 1955,100, 675.
Yu. V. Rakitin and V. T. Kalinnikov,Sovremennaya magnetokhimiya [Modern Magnetochemistry], Nauka, St. Petersburg, 1994 (in Russian).
U. Turpeinen, R. Hamalainen, and J. Reedijk,Polyhedron, 1987,6, 1603.
D. Volkmer, B. Hommerich, K. Griesar, W. Haase, and B. Krebs,Inorg. Chem., 1996,35, 3792.
J. H. van Vleck,The Theory of Electronic and Magnetic Susceptibilities, Oxford University Press, London, 1932.
F. Loret, J. Sletten, R. Ruiz, M. Julve, and M. Verdaguer,Inorg. Chem., 1992,31, 778.
V. M. Novotortsev, Ph. D. Thesis, Moscow Physicotechnical Institute, Moscow, 1974 (in Russian).
N. Walker and D. Stuart,Acta Crystallogr., 1983,A39, 158.
G. M. Sheldrick, inCrystallographic Computering 3: Data Collection, Structure Determination, Proteins, and Databases, New York, 1985, 175.
Author information
Authors and Affiliations
Additional information
Translated fromIzvestiya Akademii Nauk. Seriya Khimicheskaya, No. 4, pp. 725–738, April, 1998.
Rights and permissions
About this article
Cite this article
Eremenko, I.L., Golubnichaya, M.A., Nefedov, S.E. et al. Synthesis, structures, and magnetic properties of binuclear carboxylate complexes with NiII and NiIII atoms. Russ Chem Bull 47, 704–718 (1998). https://doi.org/10.1007/BF02495984
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02495984