Abstract
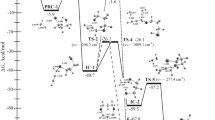
The plausible formation of 3-methylthio-1-phenyl-2-propene-1-thione and 3-methylthio-3-phenyl-2-propenethial in the reaction of 3-methylthio-3-phenyl-2-propene-1-N,N-dimethyliminium iodide with hydrogen sulfide was shown in the framework of semiempirical AM1 and PM3 methods. In polar media, these products can be trimerized into four types of structures. The kind of trimer depends on the shift of the equilibrium in the 3-methylthio-3-phenyl-2-propenethial ⇄ 3-methylthio-1-phenyl-2-propene-1-thione mixture. Each of the four types of trimers is characterized by the presence of three nearly planar sixmembered pseudo-chelate rings. The possibility of obtaining derivatives of 1,2-dithiolane (the basic fragment of lipoic acid) from the reaction products in nonpolar solvents was considered.
Similar content being viewed by others
References
L. V. Timokhina, G. M. Panova, and M. G. Voronkov,Tez. dokl. 19-i Vseros. konf. po khimii and tekhnologii organ soed. sery [Abstr. 19th All-Russ. Conf. on Chem. and Technol. of Org. Compds. of Sulfur], Kazan' (Russian), 1995, 11 (in Russian).
M. J. S. Dewar, E. G. Zoebisch, E. F. Healy, and J. J. P. Stewart,J. Am. Chem. Soc., 1985,107, 3902.
M. J. S. Dewar and E. G. Zoebisch,J. Mol. Struct., 1988,180, 1.
J. J. P. Stewart,J. Comput. Chem., 1989,10, 209.
G. E. Chudinov, D. V. Napolov, and M. V. Basilevsky,Chem. Phys., 1992,160, 41.
M. V. Bazilevsky, G. E. Chudinov, and D. V. Napolov,Khim. Fiz., 1992,11, 691 [Sov. Chem. Phys., 1992,11 (Engl. Transl.)].
M. V. Basilevsky, G. E. Chudinov, D. V. Napolov, and L. M. Timofeeva,Chem. Phys., 1993,173, 345.
J. L. Kice, inSulfur in Organic and Inorganic Chemistry, Ed. A. Senning, Dekker, New York, 1971,1, Ch. 6.
G. Bergson,Ark. Kemi., 1962,19, 265;Chem. Abstrs., 1963,58, 3292.
A. L. Fluharty inThe Chemistry of the Thiol Group, Part 2, Ed. S. Patai, Interscience, London, 1974, Ch. 13.
L. J. Reed, inComprehensive Biochemistry, Eds. M. Florkin and E. N. Stotz, Elsevier, Amsterdam, 1966,14, 99.
Author information
Authors and Affiliations
Additional information
Translated fromIzvestiya Akademii Nauk. Seriya Khimicheskaya, No. 7, pp. 1273–1277, July, 1997.
Rights and permissions
About this article
Cite this article
Shagun, V.A., Timokhina, L.V. & Panova, G.M. Quantum-chemical study of possible structural transformations in 3-methylthio-3-phenyl-2-propenethial. Russ Chem Bull 46, 1216–1220 (1997). https://doi.org/10.1007/BF02495916
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02495916