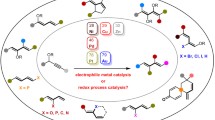
Abstract
Partial acylation of (R,S)-3,7-dimethyloctan-1-ol (1) and (R,S)-7-methoxy-3,7-dimethyloctan-1-ol (2) with vinyl acetate catalyzed by the lipase fromCandida cylindracea affords in good yields the correspondingS-configured acetates with 92–98% enantiomeric excess (ee). Under similar conditions, racemic α-cyclogeraniol (3), drim-7-en-11-ol, methyl 4-(3-hydroxy-2-methylpropyl)benzoate, and its η6-chromium(tricarbonyl) complex (6) are acylated with rather poor (and, for the two latter, opposite) enantioselectivity, whereas (R,S)-2,4∶3,5-di-O-benzylidenexylitol remains unaffected. Racemic isoborneol (8) and 2-nitro-1-phenylethanol also remain almost or completely unconverted. Attempts to perform enantioselective acylation of alcohols 3 and 8 with Ac2O in the presence of porcine pancreatic lipase (PPL) proved equally unsuccessful. By contrast, the PPL-catalyzed acylation of alcohol 6 with vinyl acetate at 17% conversion affords the levorotatory acetate (S)-6a withca. 100%ee. PPL-Mediated partial acylation of (R,S)-pantolactone with Ac2O, followed by mild deacylation of the resultingR acetate, gives (R)-(-)-pantolactone of 97% enantiomeric purity in 60% overall yield.
Similar content being viewed by others
References
C. -H. Wong and G. M. Whitesides, inEnzymes in Synthetic Organic Chemistry (Tetrahedron Organic Chemistry Series,12) Elsevier/Redwood Books, Trowbridge (UK), 1995, 70–108; K. Faber and S. Riva, inBiotransformations in Organic Chemistry: A Textbook, 2nd Ed, Springer, Berlin, 1995, 278; A. M. Klibanov,Acc. Chem. Res., 1990,23, 114; M. Murata, H. Ebike, and K. Achiwa,J. Synth. Org. Chem. Jpn., 1991,49, 1127; E. Santaniello, P. Ferraboschi, P. Grisenti, and A. Manzzocchi,Chem. Rev., 1992,92, 1071; F. Theil,Chem. Rev., 1995,95, 2203.
D. Bianchi, P. Cesti, and E. Battistel,J. Org. Chem., 1988,53, 5531.
Y. -F. Wang and C. -H. Wong,J. Org. Chem., 1988,53, 3127; K. Laumen, D. Brietgoff, and M. P. Schneider,J. Chem. Soc., Chem. Commun., 1988, 1459.
E. P. Serebryakov, G. M. Zhdankina, G. V. Kryshtal, M. V. Mavrov, and C. H. Nguyen,Izv. Akad. Nauk SSSR, Ser. Khim., 1991, 842 [Bull. Acad. Sci. USSR, Div. Chem. Sci., 1991,40, 739 (Engl. Transl.)]; G. D. Gamalevich and E. P. Serebryakov,Izv. Akad. Nauk, Ser. Khim., 1993, 342 [Russ. Chem. Bull., 1993,42, 300 (Engl. Transl.)].
C. A. Henrick, R. J. Anderson, G. B. Staal, and G. F. Ludvick,J. Agric. Food Chem., 1978,26, 542.
K. Mori, H. Harada, P. Zagatti, A. Cork, and D. R. Hall,Liebigs Ann. Chem., 1991, 259.
T. Fujisawa, T. Sato, and K. Ohshi,Tetrahedron Lett., 1981,22, 4823; N. Cohen, W. R. Eichel, R. J. Lopresti, C. Neukom, and G. Saucy,J. Org. Chem., 1976,41, 3505; M. Schmid, F. Geber, and G. Hirth,Helv. Chim. Acta, 1982,65, 684.
K. Mori, M. Amaike, and M. Itoh,Tetrahedron, 1993,49, 1871.
H. Maturana, J. Sierra, J. Lopes, and M. Cotes,Synth. Commun., 1984,14, 661.
K. Gustafson and R. J. Andersen,Tetrahedron, 1985,41, 1101; M. L. Oyarzun, M. Cortes, and J. Sierra,Synth. Commun., 1982,12, 951.
G. D. Gamalevich, A. V. Ignatenko, E. P. Serebryakov. and N. E. Voishvillo,Izv. Akad. Nauk, Ser. Khim., 1995. 761 [Russ. Chem. Bull., 1995,44, 743 (Engl. Transl.)].
E. P. Serebryakov, G. D. Gamalevich, A. V. Strakhov, and A. A. Vasil'ev,Mendeleev Commun., 1995, 175.
Eur. Pat. Appl. EP 560284;Chem. Abstrs., 1993,119, No. 195686i; H. M. Park, D. M. Piatak, J. R. Peterson, and A. M. Clark,Can. J. Chem., 1992,70, 1662; Eur. Pat. App. EP 464769;Chem Abstrs., 1992,116, No. 194800t.
C. -S. Chen, Y. Fujimoto, G. Girdaukas, and C. J. Sih,J. Am. Chem. Soc., 1982,104, 7294 C. -S. Chen and C. J. Sih,Angew. Chem., Int. Ed. Engl., 1989,28, 695.
J. Kenyon and H. E. M. Priston,J. Chem. Soc., 1925,127, 1472.
V. S. Parvar, A. K. Prasad, P. K. Singh, and S. Gupta,Tetrahedron: Asymmetry, 1992,3, 1395; S. Sankaranarayanan, A. Sharma, B. A. Kulkarni, and S. Chattopadhyay,J. Org. Chem., 1995,60, 4251.
P. A. Fitzpatrick and A. M. Klibanov,J. Am. Chem. Soc., 1991,113, 3166.
Ger. Offen. DE 4005150, 1991;Chem. Abstrs., 1992,116, 19736; Eup. Pat. Appl. EP 439779, 1991;Chem. Abstrs., 1992,116, 57573.
Eup. Pat. Appl. EP 442497;Chem. Abstrs., 1992,117. 232211; Eup. Pat. Appl. EP 507278, 1992;Chem. Abstrs., 1993,118, 2965.
Y. Naoshima, Y. Munakata, S. Yoshida, and A. Funai,J. Chem. Soc., Perkin Trans. I, 1991, 549.
P. F. Vlad, N. D. Ungur, V. Kh. Nguen, and V. B. Perutsky,Izv. Akad. Nauk, Ser. Khim., 1995, 2494 [Russ. Chem. Bull., 1995,44, 2390 (Engl. Transl.)].
R. Buchker, R. Egli, H. Redel-Wied, Cs. Tscharner, C. H. Eugster, G. Uhde, and G. Ohloff,Helv. Chim. Acta, 1973,56, 2548.
H. Mayer and A. Rüttimann,Helv. Chim. Acta, 1980,63, 1451.
A. G. Andrews, G. Borch, and S. Liaaen-Jensen,Acta Chem. Scand., 1984,B38, 871.
H. H. Appel, C. J. Brooks, and K. H. Overton,J. Chem. Soc., 1959, 3322.
S. Huneck,Z. Naturforsch., 1967,22b, 104; S. W. Pelletier, S. Laiŝić, Y. Ohtsuka, and Z. Djarmati,J. Org. Chem., 1975,40, 1607.
J. A. Hueso-Rodrigues and B. Rodrigues,Tetrahedron, 1989,45, 1569.
M. S. Nair and A. T. Aniekumar,Tetrahedron: Asymmetry, 1996,7, 511.
E. P. Serebryakov and G. D. Gamalevich,Mendeleev Commun., 1996,6, 221.
Y. Asahina, M. Ishidate, and T. Sano,Ber., 1936,69, 343; E. Avela,Ann. Acad. Sci. Fennicae, Ser. A II, 1956, No. 77, 72.
R. Kuhn and Th. Wieland,Ber., 1940,73, 971.
S. A. Harris, G. A. Boyack, and K. Folkers,J. Am. Chem. Soc., 1941,63, 2662.
Hung. Pat. 177197 (P);Chem. Abstrs., 1981,95, 204217.
M. L. Wolfrom and E. J. Kohn,J. Am. Chem. Soc., 1942,64, 1739.
X. Beabe, N. E. Schore, and M. J. Kurth,J. Org. Chem., 1995,60, 4196.
J. A. Riddick and W. B. Bunger,Techniques of Chemistry. 2. Organic Solvents, Wiley, New York, 1971.
J. A. Dale and H. S. Mosher,J. Am. Chem. Soc., 1973,95, 512.
N. Kalyanam and D. A. Lightner,Tetrahedron Lett., 1979, 415.
Author information
Authors and Affiliations
Additional information
Translated fromIzvestiya Akademii Nauk. Seriya Khimicheskaya, No. 1, pp. 175–186, January, 1997.
Rights and permissions
About this article
Cite this article
Gamalevich, G.D., Serebryakov, E.P. Enantioselectivity of enzymatic acylation of some structurally various racemic alcohols in anhydrous aprotic media. Russ Chem Bull 46, 171–183 (1997). https://doi.org/10.1007/BF02495369
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02495369