Abstract
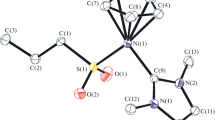
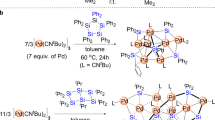
Reactions of Cp2Cr2(SCMe3)2S (1) with rhenium complexes (CO)(NO)Re(PR3)2X2 [R=Et, X=Cl (7a); R=Et, X=O3SCF3 (7b); R=OMe, X=O3SCF3 (7c)] containing strongly bound phosphine ligands and with Pd(PPri 3)2Cl2 (8) containing bulky P donors were studied. The reaction between compounds1 and7a does not occur in various solvents within a temperature range of 22–80 °C. Interaction of1 with triflat derivatives7b and7c yields the paramagnetic tetrahedral homonuclear cationic cluster Cp4Cr4S4 +O3SCF3 − (10) and the binuclear methylated complex Cp2Cr2(SCMe3)2(SMe)+O3SCF3 − (11), respectively. The reaction of compound1 with8 affords the antiferromagnetic heteronuclear cluster Cp2Cr2(SCMe3)S2PdCl(PPri 3) (12). The structure of the core of12 is analogous to the structures of the rhodium-containing complexes Cp2Cr2(μ-SCMe3)(μ3-S)2RhL2. Although compound8 reacts with Fe3S2(CO)9 (5), the major products are the homometallic trinuclear clusters Fe3S2(CO)8(PPri 3) (14) (as a mixture of isomers) and Fe3S2(CO)7(PPri 3)2 (15), whereas the heteronuclear complex (CO)6Fe2S2Pd(PPri 3)2 (16) was found only in trace amounts. The reasons for the difference in the reactivities of the rhenium and palladium derivatives toward compounds1 and5 are discussed. The structures of complexes10 (two crystal modifications),11, 12, 15, and16 were established by X-ray structural analysis of the single crystals.
Similar content being viewed by others
References
I. L. Eremenko, H. Berke, B. I. Kolobkov, and V. M. Novotortsev,Organometallics, 1994,13, 244.
I. L. Eremenko, H. Berke, A. A. H. van der Zejden, and V. M. Novotortsev,J. Organomet. Chem., 1994,471, 123.
A. A. Pasynskii, I. L. Eremenko, G. Sh. Gasanov, O. G. Ellert, V. M. Novotortsev, Yu. V. Rakitin, T. Kh. Kurbanov, V. T. Kalinnikov, Yu. T. Struchkov, and V. E. Shklover,Polyhedron, 1984,3, 775.
A. A. Pasynskii, B. I. Kolobkov, I. L. Eremenko, S. E. Nefedov, S. B. Katser, and M. A. Porai-Koshits,Zh. Neorg. Khim., 1992,37, 563 [Russ. J. Inorg. Chem., 1992,37 (Engl. Transl.)].
A. A. Pasynskii, I. L. Eremenko, Yu. V. Rakitin, V. M. Novotortsev, V. T. Kalinnikov, G. G. Aleksandrov, and Yu. T. Struchkov,J. Organomet. Chem., 1979,165, 57.
D. Veghini and H. Berke,Chimia, 1993,47, 284.
W. Chen, L. Y. Goh, R. F. Bryan, and E. Sinn,Acta Crystallogr., 1986,C42, 796.
A. A. Pasynskii, I. L. Eremenko, A. S. Katugin, S. E. Nefedov, G. Sh. Gasanov, B. Orazsakhatov, O. G. Ellert, V. E. Shklover, and Yu. T. Struchkov,Metalloorg. Khim., 1988,1, 172 [Organomet. Chem. USSR, 1988,1 (Engl. Transl.)].
P. D. Williams and M. D. Curtis,Inorg. Chem., 1986,25, 4562.
Yu. L. Slovokhotov, M. Yu. Antipin, R. G. Gerr, A. I. Yanovsky, and Yu. T. Struchkov,Dokl. Akad. Nauk SSSR, 1985,285, 1413 [Dokl. Chem., 1985,285 (Engl. Transl.)].
M. Yu. Antipin, Yu. L. Slovokhotov, and Yu. T. Struchkov,Dokl. Akad. Nauk SSSR, 1987,287, 1143 [Dokl. Chem., 1987,287 (Engl. Transl.)].
J. H. van Vleck,The Theory of Electronic and Magnetic Susceptibilities, Oxford University Press, London, 1932.
Yu. V. Rakitin and V. T. Kalinnikov,Sovremennaya magnetokhimiya [Modern Magnetochemistry], Nauka, St. Petersburg, 1994, (in Russian).
A. A. Pasynskii, I. L. Eremenko, B. Orzsakhatov, Yu. V. Rakitin, V. M. Novotortsev, O. G. Ellert, and V. T. Kalinnikov,Inorg. Chem. Acta, 1988,39, 91.
A. A. Pasynskii, I. L. Eremenko, V. R. Zalmanovich, V. V. Kaverin, B. Orazsakhatov, V. M. Novotortsev, O. G. Ellert, A. I. Yanovsky, and Yu. T. Struchkov,J. Organomet. Chem., 1988,356, 57.
A. A. Pasynskii, I. L. Eremenko, V. V. Zalmanovich, V. V. Kaverin, B. Orazsakhatov, S. E. Nefedov, A. I. Yanovsky, and Yu. T. Struchkov,J. Organomet. Chem., 1991,414, 55.
C. A. Tolman,Chem. Rev., 1977,77, 331.
C. H. Wei and L. F. Dahl,Inorg. Chem., 1965,4, 493.
S. Aime, L. Milone, R. Rosetti, and P. L. Stanghellini,J. Chem. Soc., Dalton Trans., 1980, 46.
N. Walker and D. Stuart,Acta Crystallogr., 1983,A39, 158.
G. M. Sheldrick, inCrystallographic Computing 3. Data Collection, Structure Determination, Proteins and Databases, New York, 1985, 175.
Author information
Authors and Affiliations
Additional information
For Part 4, see I. L. Eremenko, S. E. Nefedov, H. Berke, B. I. Kolobkov, and V. M. Novotortsev,Organometallics, 1995,14, 1132.
Translated fromIzvestiya Akademii Nauk. Seriya Khimicheskaya, No. 1, pp. 141–152, January, 1997.
Rights and permissions
About this article
Cite this article
Eremenko, I.L., Nefedov, S.E., Veghini, D.A. et al. Formation of antiferromagnetic thiolate-bridged and sulfide-bridged complexes. Russ Chem Bull 46, 137–148 (1997). https://doi.org/10.1007/BF02495363
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02495363