Abstract
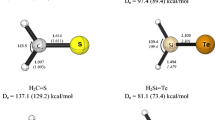
The structures of 6,6-dimethyl-6-silafulvene C5H4SiMe2 (3), its donor-acceptor complex with ammonia. C5H4SiMe2·NH3, dimethylfulvene, a number of cyclopentadienylides, methylenetrimethylphosphorane (6), and silicon-containing organophosphorus betaine−C5H4SiMe2CH2PMe3 + (13), the product of nucleophilic addition of6 to3, were calculated using the density functional approach. For compound13, the potential energy minimum corresponds to the conformation withgauche-arrangement of the cyclopentadienyl anionie and trimethylphosphonium cationic centers and a C−Si−C−P dihedral angle of 30.5°, which is due to the Coulomb attraction between these centers. According to calculations, betaine13 is rather stable toward decomposition into3 and6 (ΔH o=42 kcal mol−1, ΔG Δ=30 kcal mol−1). The main channel of thermal decomposition of compound13 involves an intramolecular nucleophilic substitution, which proceeds with elimination of trimethylphosphine and results in 1,1-dimethyl-1-silaspiro[2,4]hepta-4,6-diene, which then undergoes a ready and irreversible isomerization into 6,6-dimethyl-6-silabicyclo[3.2.0]hepta-1,3-diene owing to the [1.5]-sigmatropic shift of the C−Si bond.
Similar content being viewed by others
References
I. V. Borisova, N. N. Zemlyanskii, V. N. Khrustalev, Yu. A. Ustynyuk, and E. A. Chernyshev,Izv. Akad Nauk, Ser. Khim., 2000, 1594 [Russ. Chem. Bull. Int. Ed. Engl., 2000,49, 1583].
A. G. Brook and A. M. Brook,Adv. Organomet. Chem., 1996,39, 71.
I. Hemme and U. Klingebiel,Adv. Organomet. Chem., 1996,39, 159.
V. N. Knabashesku, K. N. Kudin, J. Tamas, S. E. Boganov, J. L. Margrave, and O. M. Nefedov,J. Am. Chem. Soc., 1998,120, 5005.
M. Driess,Adv. Organomet. Chem., 1996,39, 193.
R. Okazaki and R. West,Adv. Organomet. Chem., 1996,39, 232.
N. Wiberg, G. Wagner, G. Reber, and G. MüllerOrganometallics, 1987,6, 35.
N. Wiberg and H. Kopf,J. Organomet. Chem., 1986,315, 9.
P. Arya, J. Boyer, F. Carre, R. Corriu, G. Lanneau, J. Lapassett, M. Perrot, and C. Prim,Angew. Chem., Int. Ed. Engl., 1989,28, 1016.
Yu. A. Ustynyuk, P. I. Zakharov, A. A. Azizov, and G. A. Schembelov,J. Organomet. Chem., 1975,96, 195.
Y. Nakadaira, H. Sakaba, and H. Sakurai,Chem. Lett., 1980, 1071.
T. J. Barton, G. T. Burns, E. V. Arnold, and J. Clarly,Tetrshedron Lett., 1981,22, 7.
N. N. Zemlyanskii, I. V. Beletskaya, V. K. Bel'skii, Yu. A. Ustynyuk, and I. P. Beletskaya,Izv. Akad Nauk SSSR, Ser. Khim., 1983, 953 [Bull. Acad. Sci. USSR. Div. Chem. Sci., 1983,32, 863 (Engl. Transl.)].
I. P. Beletskaya and N. N. Zemlyansky, inAdv. Organomet. Chem., Ed. O. A. Reutov, Mir, Moscow, 1984, a) p. 30: b) p. 88.
I. V. Borisova, N. N. Zemlyansky, V. K. Belsky N. D. Kolosova, A. N. Sobolev, Yu. A. Ustynyuk, and I. P. Beletskaya,J. Chem. Soc., Chem. Commun., 1982, 1090.
I. V. Borisova, N. N. Zemlyanskii, Yu. A. Ustynyuk, I. P. Beletskaya, and E. A. Chernyshev,Metalloorg. Khim., 1992,5, 537 [Organomet. Chem. USSR, 1992,5, 262 (Engl. Transl.)].
I. V. Borisova, N. N. Zemlyanskii, Yu. N. Luzikov Yu. A. Ustynyuk, V. K. Bel'skii, M. M. Shtern, and I. P. Beletskaya,Dokl. Akad. Nauk SSSR, 1983,269, 369 [Dokl. Chem., 1983 (Engl. Transl.)].
I. V. Borisova, N. N. Zemlyanskii, A. K. Shestakova, and Yu. A. Ustynyuk,Izv. Akad. Nauk. Ser. Khim., 1993, 2140 [Russ. Chem. Bull., 1993,43, 2137 (Engl. Transl.)].
I. V. Borisova, N. N. Zemlyanskii, Yu. A. Ustynyuk, V. N. Khrustalev, S. V. Lindeman, and Yu. T. Struchkov,Izv. Akad. Nauk, Ser. Khim., 1994, 339 [Russ. Chem. Bull., 1994,43, 318 (Engl. Transl.)].
N. N. Zemlyanskii, I. V. Borisova, A. K. Shestakova, Yu. A. Ustynyuk, and E. A. Chernyshev,Izv. Akad. Nauk, Ser. Khim., 1994, 2246 [Russ. Chem. Bull., 1994,43, 2116 (Engl. Transl.)].
I. V. Borisova, N. N. Zemlyansky, A. K. Shestakova, and Yu. A. Ustynyuk.Mendeleev Commun., 1996, 90: 229.
I. V. Borisova, N. N. Zemlyanskii, A. K. Shestakova, Yu. A. Ustynyuk, and E. A. Chernyshev,Izv. Akad. Nauk, Ser. Khim., 2000, 922 [Russ. Chem. Bull. Int. Ed. Engl., 2000,49, 920].
V. N. Khrustalev, N. N. Zemlyanskii, I. V. Borisova, Yu. A. Ustynyuk, and E. A. Chernyshev,Izv. Akad. Nauk, Ser. Khim., 2000, 931 [Russ. Chem. Bull., Int. Ed. Engl., 2000,49, 929].
I. V. Borisova, N. N. Zemlyanskii, A. K. Shestakova, V. N. Khrustalev, Yu. A. Ustynyuk, and E. A. Chernyshev,Izv. Akad. Nauk, Ser. Khim., 2000, 935 [Russ. Chem. Bull., Int. Ed. Engl., 2000,49, 933].
D. N. Laikov,Chem. Phys. Lett., 1997,281, 151.
J. P. Perdew, K. Burke, and M. Ernzerhof,Phys. Rev. Lett., 1996,77, 3865.
O. I. Trifonova, E. A. Ochertyanova, N. G. Akhmedov, V. A. Roznyatovsky, D. A. Laikov, N. A. Ustynyuk, and Yu. A. Ustynyuk,Inorg. Chim. Acta. 1998, 280: 328.
Yu. A. Ustynyuk, L. Yu. Ustynyuk, D. N. Laikov, and V. V. Lunin,Izv. Akad. Nauk. Ser. Khim., 1999, 2248 [Russ. Chem. Bull., 1999,48, 2222 (Engl. Transl.)]: b) Yu. A. Ustynyuk, L. Yu. Ustynyuk, D. N. Laikov, and V. V. Lunin,J. Organomet. Chem., 2000,597, 182.
N. W. Mitzel, D. H. Brown, S. Parsons, P. T. Brain, C. R. Pulham, and D. W. H. Rankin,Angew. Chem., Int. Ed. Engl., 1998,37, 1670.
H.-J. Bestmann and R. Zimmermann.Methoden der Organischen Chemie (Houben-Weyl-Müller), 1982,E1.
M. Driess and H. Grutzmacher,Angew. Chem., Int. Ed. Engl., 1996,35, 828.
N. Norman and B. Post.Acta Crystallogr., 1961,14, 503.
V. G. Andrianov and Yu. T. Struchkov,Izv. Akad Nauk SSSR, Ser. Khim., 1977, 687 [Bull. Acad. Sci. USSR. Div. Chem. Sci., 1977,26, 687 (Engl. Transl.)].
H. L. Ammon, G. L. Wheeler, and P. H. Watts, Jr.,J. Am. Chem. Soc., 1973,95, 6158.
Author information
Authors and Affiliations
Additional information
For Part 4, see Ref. 1.
Published inIzvestiya Akademii Nauk. Seriya Khimicheskaya, No. 11, pp. 1850–1857, November, 2000.
Rights and permissions
About this article
Cite this article
Nechaev, M.S., Borisova, I.V., Zemlyanskii, N.N. et al. Heteroorganic betaines. Russ Chem Bull 49, 1823–1830 (2000). https://doi.org/10.1007/BF02494917
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02494917