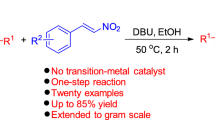
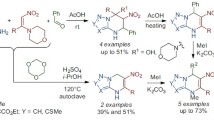
Abstract
A number of 1,4-dihydropyridazines and pyridazines were prepared by the Diels-Alder reaction with an inverse electron demand from cyclic heterodiene systems, 3,6-bis(3,5-dimethyl-4-R-pyrazol-1-yl)-1,2,4,5-tetrazines, and some enamines as well as from 4-vinylpyridine, butyl vinyl ether, phenylacetylene, and acrylamide. The reaction of 3,6-bis(3,5-dimethylpyrazol-1-yl)-1,2,4,5-tetrazine with styrene afforded 4,5-dihydropyridazine, which was readily oxidized by atmospheric oxygen to form the corresponding pyridazine. Electron-withdrawing substituents (Br or Cl) in the pyrazole rings accelerate [4+2]-cycloaddition. When heated, 1,4-dihydropyridazines, which were synthesized from tetrazines and enamines, eliminated amine to give pyridazines. The reactivities of tetrazines were evaluated by quantum-chemical methods.
Similar content being viewed by others
References
R. A. Carboni and R. V. Lindsey,J. Am. Chem. Soc., 1959,81, 4342.
E. G. Kovalev, I. Ya. Postovskii, G. L. Rusinov, and I. L. Shegal,Khim. Geterotsikl. Soedin., 1981,11, 1462 [Chem. Heterocycl. Compd., 1981,11 (Engl. Transl.)].
J. Sauer,Khim. Geterotsikl. Soedin., 1995,25, 1307 [Chem. Heterocycl. Compd., 1995,25 (Engl. Transl.)].
D. L. Boger and S. M. Sakya,J. Org. Chem., 1988,53, 1415.
S. M. Sakya, K. K. Groskopf, and D. L. Boger,Tetrahedron Lett., 1997,38, 3805.
J. Sauer, inComprehensive Heterocycl. Chem. II, 1996,6, 901.
G. L. Rusinov, N. I. Latosh, I. N. Ganebnych, R. I. Ishmetova, and O. N. Chupakhin,16th International Congress of Heterocyclic Chemistry. Abstracts Book, Bozeman, USA, 1997, 196.
N. I. Latosh, G. L. Rusinov, and E. G. Polovinko,International Memorial I. Postovsky Conference on Organic Chemistry. Abstracts Book, Ekaterinburg, Russia, 1998, 90.
C. Glidewell, P. Lightfoot, B. Royles, and D. Smith,J. Chem. Soc., Perkin Trans. 2, 1997, 1167.
M. Avram, I. G. Dinulescu, E. Marica, and C. D. Nenitzescu,Chem. Ber., 1962,95, 2250.
K. Nakanishi,Infrared Absorption Spectroscopy, Holden-Day, San Francisco, 1962.
J. W. Wijnen, S. Zavarise, J. B. F. N. Engberts, and M. Charton,J. Org. Chem., 1996,61, 2001.
M. D. Coburn, G. A. Buntain, B. W. Harris, M. A. Hiskey, K.-Y. Lee, and D. G. Ott,J. Heterocycl. Chem., 1991,23, 2049.
S. Hünig and W. Lendle,Chem. Ber., 1960,93, 909.
Author information
Authors and Affiliations
Additional information
Translated fromIzvestiya Akademii Nauk. Seriya Khimicheskaya, No. 2, pp. 354–360, February, 2000.
Rights and permissions
About this article
Cite this article
Rusinov, G.L., Ishmetova, R.I., Latosh, N.I. et al. [4+2]-Cycloaddition of 3,6-bis(3,5-dimethyl-4-R-pyrazol-1-yl)-1,2,4,5-tetrazines with alkenes. Russ Chem Bull 49, 355–362 (2000). https://doi.org/10.1007/BF02494688
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02494688