Abstract
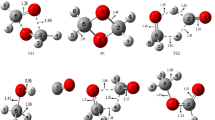
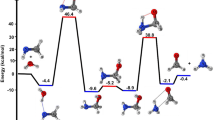
The gradient pathways of the reactions of nucleophilic addition of H2O and HF molecules to formaldehyde in the gas phase and in the XH…H2CO…HC(O)OH complex (X=OH, F) were calculated by theab initio RHF/6-31G**, MP2(fc)/6-31G**, and MP2(full)/6-311++G** methods. Both reactions proceed concertedly. The formation of H-bonded bimolecular pre-reaction complexes is the initial stage of the gas-phase reactions; at the same time, no indications of the formation of stable π-complexes were found on the potential energy surfaces of systems under study. The calculated energy barriers to the gasphase reactions exceed 40 kcal mol−1, while those to reactions in the complex XH…H2CO…HC(O)OH (X=OH, F) become more than halved.
Similar content being viewed by others
References
W. P. Jencks,Catalysis in Chemistry and Biochemistry, McGraw-Hill, New York, 1969.
A. J. Kirby,Angew. Chem., Int. Ed. Engl., 1996,35, 707;Adv. Phys. Org. Chem. 1994,29, 87.
V. I. Minkin, B. Ya. Simkin, and R. M. Minyaev,Quantum Chemistry of Organic Compounds. Mechanisms of Reactions, Springer-Verlag, Berlin, 1990, 270 pp.
H. B. Burgi and J. D. Dunitz,Acc. Chem. Res., 1983,16, 153.
Y.-T. Chang and G. H. Loew,J. Am. Chem. Soc., 1994,116, 3548.
R. M. Minyaev,Izv. Akad. Nauk, Ser. Khim., 1998, 13 [Russ. Chem. Bull., 1998,47, 8 (Engl. Transl.)].
A. Fiedler, D. Schroder, H. Schwarz, B. L. Tjelta, and P. B. Armentrout,J. Am. Chem. Soc., 1996,118, 5047.
O. N. Ventura, E. L. Coitino, A. Lledos, and J. Bertran,J. Comput. Chem., 1992,13, 1037.
R. M. Minyaev,Usp. Khim., 1994,63, 939 [Russ. Chem. Rev., 1994,63, 883 (Engl. Transl.)].
H. Yamataka, S. Nagase, T. Ando, and T. Nanafusa,J. Am. Chem. Soc., 1986,108, 601.
I. H. Williams,Chem. Soc. Rev., 1993,22, 277.
C. Kozmutza, E. M. Evleth, and E. Kapuy,J. Mol. Struct. (Theochem.), 1991,233, 139.
F. Leng, R. Savkur, I. Fokt, T. Przewloka, W. Priebe, and J. B. Chaires,J. Am. Chem. Soc., 1996,118, 4731 (and references cited therein).
F. A. Baiocchi and W. Klemperer,J. Chem. Phys., 1983,78, 3509.
L. Schriver, O. Abdelaoui, and A. Schriver,J. Chem. Phys., 1992,96, 8069.
O. Abdelaoui, L. Schriver, and A. Schriver,J. Mol. Struct., 1992,268, 335.
A. Nowek and J. Leszczynski,J. Chem. Phys., 1996,104, 1441.
T. Yamabe, C.-D. Zhao, M. Koizumi, A. Tachibana, and K. Fukui,Can. J. Chem., 1985,63, 1532.
K. Yamashita, T. Yamabe, and K. Fukui,Theor. Chim. Acta, 1982,60, 523.
J. D. Madura and W. L. Jorgensen,J. Am. Chem. Soc., 1986,108, 2517.
J. B. Foresman and E. Frisch,Exploring Chemistry with Electronic Structure Methods, 2nd Ed., Gaussian, Inc., Pittsburgh, 1996, 302 pp.
M. J. Frish, G. W. Trucks, H. B. Schlegel, P. M. W. Gill, B. G. Johnson, M. A. Robb, J. R. Cheeseman, T. A. Keith, G. A. Petersson, J. A. Montgomery, K. Raghavachari, M. A. AlLaham, V. G. Zakrzewski, J. V. Ortiz, J. B. Foresman, C. Y. Peng, P. Y. Ayala, W. Chen, M. W. Wong, J. L. Andres, E. S. Replogle, R. Gomperts, R. L. Martin, D. J. Fox, J. S. Binkley, D. J. Defrees, J. Baker, J. P. Stewart, M. Head-Gordon, C. Gonzalez, and J. A. Pople,GAUSSIAN-94, Revision B.3, Gaussian, Inc., Pittsburgh (PA), 1995.
M. W. Schmidt, K. K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Koseki, N. Matsunaga, K. A. Nguyen, S. J. Su, T. L. Windus, M. Dupuis, and J. A. Montgomery,J. Comput. Chem., 1993,14, 1347.
K. S. Krasnov, N. V. Filippenko, V. A. Bobkova, N. L. Lebedeva, E. V. Morozov, T. I. Ustinova, and G. A. Romanova,Molekulyarnye postoyannye neorganicheskikh soedinenii [Molecular Constants of Inorganic Compounds], Khimiya, Leningrad, 1979, 446 pp. (in Russian).
Y. Yamada, T. Nakagawa, K. Kuchisu, and Y. Morino,J. Mol. Spectrosc., 1971,38, 70.
J. L. Derissen,J. Mol. Struct., 1971,7, 67.
A. D. Buckingham,J. Mol. Struct., 1991,250, 111.
D. E. Reisner, R. W. Field, J. L. Kinsey, and H.-L. Dai,J. Chem. Phys., 1984,80, 5968.
O. A. Osipov, V. I. Minkin, and A. D. Garnovskii,Spravochnik po dipol'nym momentam [Handbook of Dipole Moments], Vysshaya Shkola, Moscow, 1971, 414 pp. (in Russian).
T. K. Ha, J. Makarewicz, and A. Bauder,J. Chem. Phys., 1993,97, 11415.
I. H. Williams,J. Am. Chem. Soc., 1987,109, 6299.
B. J. Nelander,J. Chem. Phys., 1980,72, 77;Chem. Phys., 1992,159, 281.
E. E. Astrup,Acta Chem. Scand., 1971,25, 1494.
A. C. Legon and D. J. Millen, inPrinciples of Molecular Recognition, Eds. A. D. Buckingham, A. C. Legon, and S. M. Roberts, Blackie Academic and Professional, London, 1993, 16.
T. Steiner, J. A. Kanter, and J. Kroon,J. Chem. Soc., Chem. Commun., 1996, 1277.
G. A. Jeffrey and W. Saenger,Hydrogen Bonding in Biological Structures, Springer-Verlag, New York, 1991, 569 pp.
R. M. Lees and J. G. Baker,J. Chem. Phys., 1968,48, 5299.
D. R. Herschbach,J. Chem. Phys., 1956,25, 358.
R. M. Minyaev and E. A. Lepin,Mendeleev Commun., 1997, 80, 189 ftp://soul.rnd.runnet.ru/pub/gorguts.zip|url
F. Hibbert,Adv. Phys. Org. Chem., 1986,22, 113.
M. C. Etter,J. Phys. Chem., 1991,95, 4601.
S. Scheiner,Acc. Chem. Res., 1994,27, 402.
Author information
Authors and Affiliations
Additional information
Translated fromIzvestiya Akademii Nauk. Seriya Khimicheskaya, No. 11, pp. 2146–2154, November, 1998.
Rights and permissions
About this article
Cite this article
Minyaev, R.M., Starikov, A.G. & Lepin, E.A. Pathways of the reactions of nucleophilic addition of H2O and HF molecules to formaldehyde in the gas phase and in the complex with formic acid:ab initio calculations. Russ Chem Bull 47, 2078–2086 (1998). https://doi.org/10.1007/BF02494259
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02494259