Abstract
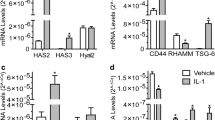
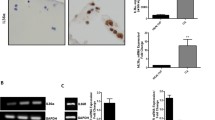
We investigated the effects of interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) on CD44 mRNA expression in cultured rabbit articular chondrocytes. High-density-suspension-cultured chondrocytes were exposed to IL-1β (0.1, 1, or 10 ng/ml) or TNF-α (0.1, 1, or 10 ng/ml). Quantitative detection of specific mRNA for CD44 was carried out by reverse transcription, polymerase chain reaction (RT-PCR). Furthermore, to determine the degradation of cartilage matrix by IL-1β and TNF-α, the concentrations of chondroitin 4-sulfate (C4S) and chondroitin 6-sulfate (C6S) released to the culture medium were measured with high-performance liquid chromatography (HPLC). CD44 mRNA expression was increased significantly in chondrocytes cultured with IL-1β, but not in the chondrocytes cultured with TNF-α. The release of chondroitin sulfates (C4S+C6S) to the culture medium was also accelerated by IL-1β but was not affected by TNF-α. These results suggest that IL-1β can promote CD44 mRNA expression, together with the degradation of the cartilage matrix, and may assist in binding hyaluronic acid (HA) and CD44 in the chondrocytes.
Similar content being viewed by others
References
Dayer JM, Demczuk S (1984) Cytokines and other mediators in rheumatoid arthritis. Springer Semin Immunopathol 7:387–413
Arend WP, Dayer JM (1990) Cytokines and cytokine inhibitors or antagonist in rheumatoid arthritis. Arthritis Rheum 33:305–315
Haynes BF, Hale LP, Patton KL, Martin ME, McCallum RM (1991) Measurement of an adhesion molecule as an indicator of inflammatory disease activity. Up-regulation of the receptor for hyaluronate (CD44) in rheumatoid arthritis. Arthritis Rheum 34:1434–1443
Hale LP, Haynes BF, McCachren SS (1995) Expression of CD44 variants in human inflammatory synovitis. J Clin Immunol 15:300–311
Takagi T, Suzuki K, Okamoto R, Mitsugi N, Saito T, Noyori K, Mitani Y, Matsukura Y, Koshino T (1994) Expression of CD44 on human articular chondrocytes. Arthritis Rheum 37:S191
Lesley J, Hyman R, Kincade PW (1993) CD44 and its interaction with extracellular matrix. Adv Immunol 54:271–335
Haynes BF, Liao H, Patton KL (1991) The transmembrane hyaluronate receptor (CD44): multiple functions, multiple forms. Cancer Cells (Cold Spring Harbor) 3:347–350
Underhill C (1992) CD44: the hyaluronan receptor. J Cell Sci 103:293–298
Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B (1990) CD44 is the principal cell surface receptor for hyaluronate. Cell 61:1303–1313
Miyake K, Underhill CB, Lesley J, Kincade PW (1990) Hyaluronate can function as a cell adhesion molecule and CD44 participates in hyaluronate recognition. J Exp Med 172:69–75
Murakami H, Horiuchi S (1994) Protective effect of high molecular weight hyaluronate on matrix of cultured chondrocytes. Jpn J Rheum Joint Surg 13:57–66
Tajima G, Murakami H, Mizusawa N, Horiuchi S (1995) Effect of high molecular hyaluronate on collagen mRNA expression in cultured articular chondrocytes. Jpn J Rheum Joint Surg 14:63–72
Shimomura Y, Yoneda T, Suzuki F (1975) Osteogenesis by chondrocytes from growth cartilage of rat rib. Calcif Tissue Res 19:179–187
Inoue H, Kato Y, Iwamoto M, Hiraki Y, Sakuda M, Suzuki F (1989) Stimulation of cartilage-matrix proteoglycan synthesis by morphologically transformed chondrocytes grown in the presence of fibroblast growth factor and transforming growth factor-beta. J Cell Physiol 138:329–337
Kato Y, Iwamoto M, Koike T, Suzuki F, Takano Y (1988) Terminal differentiation and calcification in rabbit chondrocyte cultures grown in centrifuge tubes: regulation by transforming growth factor β and serum factors. Proc Natl Acad Sci USA 85:9552–9556
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Noonan KE, Roninson IB (198) mRNA phenotyping by enzymatic amplification of randomly primed cDNA. Nucleic Acids Res 16:10366
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Gendelman HE, Friedman RM, Joe S, Baca LM, Turpin JA, Dveksler G, Meltzer MS, Dieffenbach C (1990) A selective defect of interferon-α production in human immunodeficiency virus-infected monocytes. J Exp Med 172:1433–1442
Shinmei M, Miyauchi S, Machida A, Miyazaki K (1992) Quantitation of chondroitin 4-sulfate and chondroitin 6-sulfate in pathologic joint fluid. Arthritis Rheum 35:1304–1308
Yoshida K, Miyauchi S, Kikuchi H, Tawada A, Tokuyasu K (1989) Analysis of unsaturated disaccharides from glycosaminoglycuronan by high-performance liquid chromatography. Anal Biochem 177:327–332
Toyoda H, Shinomiya K, Yamanashi S, Kosiishi I, Imanari T (1988) Microdetermination of unsaturated disaccharides produced from chondroitin sulfates in rabbit plasma by high performance liquid chromatography with fluorometric detection. Anal Sci 4:381–384
Goldstein LA, Zhou DFH, Picker LJ, Minty CN, Bargatze RF, Ding JF, Butcher EC (1989) A human lymphocyte homing receptor, the Hermes antigen, is related to cartilage proteoglycan core and link proteins. Cell 56:1063–1072
Nottenburg C, Rees G, St John T (1989) Isolation of mouse CD44 cDNA: structural features are distinct from the primate cDNA. Proc Natl Acad Sci USA 86:8521–8525
Günthert U, Hofmann M, Rudy W, Reber S, Zöller M, Haußmann I, Matzku S, Wenzel A, Ponta H, Herrlich P (1991) A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell 65:13–24
Guo Y, Liu G, Wang X, Jin D, Wu M, Ma J, Sy M (1994) Potential use of soluble CD44 in serum as indicator of tumor burden and metastasis in patients with gastric or colon cancer. Cancer Res 54:422–426
Tanabe KK, Ellis LM, Saya H (1993) Expression of CD44R1 adhesion molecule in colon carcinomas and metastases. Lancet 341:725–726
Mackay CR, Terpe H, Stauder R, Marston WL, Stark H, Günthert U (1994) Expression and modulation of CD44 variant isoforms in humans. J Cell Biol 124:71–82
Screaton GR, Bell MV, Jackson DG, Cornelis FB, Gerth U, Bell JI (1992) Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci USA 89:12160–12164
Günthert U (1993) CD44: a multitude of isoforms with diverse functions. Curr Top Microbiol Immunol 184:47–63
Coombe DR, Rider CC (1989) Lymphocyte homing receptors cloned—a role for anionic polysaccharides in lymphocyte adhesion. Immunol Today 10:289–291
Haynes BF, Telen MJ, Hale LP, Denning SM (1989) CD44—a molecule involved in leukocyte adherence and T-cell activation. Immunol Today 10:423–428
Miyake K, Medina KL, Hayashi S, Ono S, Hamaoka T, Kincade PW (1990) Monoclonal antibodies to Pgp-1/CD44 block lymphohemopoiesis in long-term bone marrow cultures. J Exp Med 171:477–488
Seth A, Gote L, Nagarkatti M, Nagarkatti PS (1991) T-cell-receptor-independent activation of cytolytic activity of cytotoxic T lymphocytes mediated through CD44 and gp90MEL-14. Proc Natl Acad Sci USA 83:7877–7881
Pardi R, Inverardi L, Bender JR (1992) Regulatory mechanisms in leukocyte adhesion: flexible receptors for sophisticated travelers. Immunol Today 13:224–230
Shimazu Y, Van Seventer GA, Siraganian R, Wahl L, Shaw S (1989) Dual role of the CD44 molecule in T cell adhesion and activation. J Immunol 143:2457–2463
Koopman G, Van Kooyk Y, De Graaff M, Meyer CJLM, Figdor CG, Pals ST (1990) Triggering of the CD44 antigen on T lymphocytes promotes T cell adhesion through the LFA-1 pathway. J Immunol 145:3589–3593
Toyama-Sorimachi N, Sorimachi H, Tobita Y, Kitamura F, Yagita H, Suzuki K, Miyasaka M (1995) A novel ligand for CD44 is serglycin, a hematopoietic cell lineage-specific proteoglycan. J Biol Chem 270:7437–7444
Schenck P, Schneider S, Miehlke R, Prehm P (1995) Synthesis and degradation of hyaluronate by synovia from patients with rheumatoid arthritis. J Rheumatol 22:400–405
Shimazu A, Jikko A, Iwamoto M, Koike T, Yan W, Okada Y, Shinmei M, Nakamura S, Kato Y (1993) Effects of hyaluronic acid on the release of proteoglycan from the cell matrix in rabbit chondrocyte cultures in the presence and absence of cytokines. Arthritis Rheum 36:247–253
Chow G, Knudson CB, Homandberg G, Knudson W (1995) Increased expression of CD44 in bovine articular chondrocytes by catabolic cellular mediators. J Biol Chem 270:27734–27741
Schnyder J, Payne T, Dinarello CA (1987) Human monocyte or recombinant interleukin 1's are specific for the secretion of a metalloproteinase from chondrocytes. J Immunol 138:496–503
Henderson B, Pettipher ER (1989) Arthritogenic actions of recombinant IL-1 and tumour necrosis factor-α in the rabbit: evidence for synergistic interactions between cytokines in vivo. Clin Exp Immunol 75:306–310
Enomoto M, Pan H, Kinoshita A, Yutani Y, Suzuki F, Takigawa M (1990) Effects of tumor necrosis factor-α on proliferation and expression of differentiated phenotypes in rabbit costal chondrocytes in culture. Calcif Tissue Int 47:145–151
Hannum CH, Wilcox CJ, Arend WP, Joslin FG, Dripps DJ, Heimdal PL, Armes LG, Sommer A, Eisenberg SP, Thompson RC (1990) Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature 343:336–340
Firestein GS, Boyle DL, Yu C, Paine MM, Whisenand TD, Zvaifler NJ, Arend WP (1994) Synovial interleukin-1 receptor antagonist and interleukin-1 balance in rheumatoid arthritis. Arthritis Rheum 37:644–652
Herrlich P, Zoller M, Pals ST, Ponta H (1993) CD44 splice variants: metastases meet lymphocytes. Immunol Today 14:395–399
Chandrasekhar S, Harvey AK, Hrubey PS, Bendele AM (1990) Arthritis induced by interleukin-1 is dependent on the site and frequency of intraarticular injection. Clin Immunol Immunopathol 55:382–400
Author information
Authors and Affiliations
About this article
Cite this article
Toba, T., Mizusawa, N., Tajima, G. et al. Upregulation of CD44 mRNA expression by interleukin-1β in cultured rabbit articular chondrocytes. J Bone Miner Metab 15, 84–93 (1997). https://doi.org/10.1007/BF02490078
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02490078