Abstract
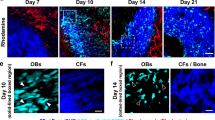
We examined the rapid formation and subsequent resorption of woven bone induced by partial ablation of rat bone marrow. On the 1st day after ablation, masses of clots occupied the region from which marrow was eliminated. On the 3rd day, alkaline phosphatase-(ALPase-) positive osteoblastic cells appeared in the vicinity of the marrow-eliminated region, forming woven bone. Other ectopic woven bone extended from the endosteal surface toward the bone marrow. Therefore, the newly formed bone originated in two different sites, the endosteal bone surface and the marrow tissues near the marrow-eliminated region. On the 7th day, numerous tartrate-resistant acid phosphatase- (TRAPase-) positive osteoclasts and ALPase-positive osteoblasts expressing the osteonectin gene indicated high activity in both formation and resorption of ectopic woven bone. On the 10th day, the ectopic bone had been markedly resorbed and replaced by bone marrow tissue as the ectopically formed woven bone had not been dynamically maintained, probably because of reduced bone formation activity. Immunoreactivity for basic fibroblast growth factor (bFGF) was indistinctly observed on osteoblastic and preosteoblastic cells on the 1st day after ablation. The fibroblastic cells in the marrow-eliminated region on the 3rd day, and both osteoblasts and preosteoblasts in the woven bone on the 7th day, showed strong immunoreactivity for bFGF. Unlike fractured cortical bone, no chondrogenesis was observed. This model appears to provide convenient material and an important clue for investigation of imbalanced bone formation and subsequent resorption.
Similar content being viewed by others
References
Amsel S, Maniatis A, Tavassoli M, Crosby WH (1969) The significance of intramedullary cancellous bone formation in the repair of bone marrow tissue. Anat Rec 164:101–112
Furusawa T (1993) The repair process of intramedullary tissue after ablation of the tibial cavity of rat. Tokyo Jikeikai Med J 108:591–607
Suva LJ, Seedor G, Endo N, Quartuccio HA, Thompson DD, Bab I, Rodan GA (1993) Pattern of gene expression following rat tidial marrow ablation. J Bone Miner Res 8:379–388
Brighton CT, Hunt RM (1991) Early histological and ultrastructural changes in medullary fracture callus. J Bone Joint Surg 6:832–847
Nakase T, Nomura S, Yoshikawa H, Hashimoto J, Hirota S, Kitamura Y, Oikawa S, Ono K, Takaoka K (1994) Transient and localized expression of bone morphogenetic protein 4 messenger RNA during fracture healing. J Bone Miner Res 9:651–659
Burstone MS (1958) Histochemical demonstration of acid phosphatase with naphtol AS-phosphate. J Natl Cancer Inst 21:523–539
Amizuka N, Karaplis AC, Henderson JE, Warshawsky H, Lipman ML, Matsuki Y, Ejiri S, Tanaka M, Izumi N, Ozawa H, Goltzman D (1996) Haploinsufficiency of parathyroid hormone-related peptide (PTHrP) results in abnormal postnatal bone development. Dev Biol 175:166–176
Matsuki Y, Nakashima N, Amizuka N, Warshawsky H, Goltzman D, Yamada KM, Yamada YA (1995) Compilation of partial sequences of randomly selected cDNA clones from the rat incisor. J Dent Res 74:307–312
Canalis E, Centrella M, McCarthy T (1988) Effects of basic fibroblast growth factor on bone formation in vitro. J Clin Invest 81:1572–1577
Globus RK, Patterson-Buckendahl P, Gospodarowicz D (1988) Regulation of bovine bone cell proliferation by fibroblast growth factor and transforming growth factor. Endocrinology 123:98–105
Noda M, Vogel R (1989) Fibroblast growth factor enhances type ß1 transforming growth factor gene expression in osteoblast-like cells. J Cell Biol 109:2529–2535
Simmons HA, Raisz LG (1991) Effects of acid and basic fibroblast growth factor and heparin on resorption of cultured fetal rat long bone. J Bone Miner Res 6:1301–1305
Wang JS, Aspenberg P (1993) Basic fibroblast growth factor and bone induction in rats. Acta Orthop Scand 64:557–561
Nagai H, Tsukuda R, Mayahara H (1995) Effects of basic fibroblast growth factor (bFGF) on bone formation in growing rats. Bone (NY) 16:367–373
Takahashi N, Akatsu T, Udagawa N, Sasaki T, Yamaguchi A, Moseley JM, Martin TJ, Suda T (1988) Osteoblastic cells are involved in osteoclast formation. Endocrinology 123:2600–2602
Takahashi N, Udagawa N, Akatsu T, Tanaka H, Shinnome M, Suda T (1991) Role of colony-stimulating factors in osteoclast development. J Bone Miner Res 6:977–985
Udagawa N, Takahashi N, Akatsu T, Sasaki T, Yamaguchi A, Kodama H, Martin TJ, Suda T (1989) The bone marrow-derived stromal cell lines MC3T3-G2/PA6 and ST2 support osteoclast-like cell differentiation in cocultures with mouse spleen cells. Endocrinology 125:1805–1813
Udagawa N, Takahashi N, Akatsu T, Tanaka H, Sasaki T, Nishihara T, Koga T, Martin TJ, Suda T (1990) The origin of osteoclasts: mature monocyte-macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-stromal cells. Proc Natl Acad Sci USA 87:7260–7264
Iwasaki M, Nakata K, Nakahara H, Nakase T, Kimura T, Kimata K, Caplan AI, Ono K (1993) Transforming growth factor ß1 stimulates chondrogenesis and inhibits osteogenesis in high density culture of periosteum-derived cells. Endocrinology 132:1603–1608
Iwasaki M, Nakahara H, Nakase T, Kimura T, Takaoka K, Caplan AI, Ono K (1994) Bone morphogenetic protein 2 stimulates osteogenesis but does not affect chondrogenesis in osteochondrogenic differentiation of periosteum-derived cells. J Bone Miner Res 9:1195–1204
Nakamura T, Hanada K, Tamura M, Shibanushi H, Tagawa M, Fukumoto S, Matsumoto T (1995) Stimulation of endosteal bone formation by systemic injections of recombinant basic fibroblast growth factor in rats. Endocrinology 136:1276–1284
Baron J, Klein KO, Yanovski JA, Novosad JA, Bacher JD, Bolander ME, Cutler GB Jr (1994) Induction of growth plate cartilage ossification by basic fibroblast growth factor. Endocrinology 135:2790–2793
Chuxia D, Wynshaw-Boris A, Zhou F, Kuo A, Leder P (1996) Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell 84:911–921
Author information
Authors and Affiliations
About this article
Cite this article
Tanaka, M., Amizuka, N., Kim, K.J. et al. Histochemical examination of ectopic bone formation induced in rat bone marrow. J Bone Miner Metab 15, 67–76 (1997). https://doi.org/10.1007/BF02490076
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02490076