Abstract
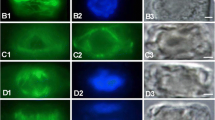
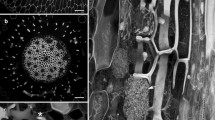
Arrangements of corticalmicrotubules (MTs) as seen in median longitudinal cryosections of shoot apices of several angiosperms and gymnosperms were studied by indirect immunofluorescence microscopy.Bryophyllum, Clethra, Helianthus, Houttuynia, Vinca (angiosperms), andPinus, Cedrus, Cedrus andGinkgo (gymnosperms) were examined. In all angiosperm apices collected during the growing season, MTs were mainly arranged anticlinally in the tunica, randomly in the corpus, and transversely in the rib meristem. This pattern of arrangements of MTs was further confirmed by electron microscopy inBryophyllum apices. In the apices of winter shoots MTs in the rib meristem were arranged randomly, indicating a seasonal change with respect to their arrangment. In all examined gymnosperm apices, populations of superficial cells showed both random and anticlinal arrangements of MTs, in contrast to those of angiosperm apices that consistently show anticlinally arranged MTs. In the shoot apices of both angiosperms and gymnosperms, cortical MTs were arranged perpendicularly to the directions of cell expansion. The significance of MTs in the maintrnance of the different architectures of shoot apices in angiosperms and gymnosperms is discussed.
Similar content being viewed by others
Abbreviations
- FITC:
-
fluorescein isothiocyanate
- MT(s):
-
microtubule(s)
References
Bachelard, E.P. andF. Wightman. 1974. Biochemical and physiological studies on dormancy release in tree buds. III. Changes in endogenous growth substances and a possible mechanism of dormancy release in overwintering vegetative buds inPopulus balsamifera Can. J. Bot.52: 1483–1489.
Bergfeld, R., V. Speth andP. Schopfer. 1988. Reorientation of microfibrils and microtubules at the outer epidermal wall of maize coleoptiles during auxin-mediated growth. Botanica Acta101: 57–67.
Epstein, E. 1972. Mineral Nutrition of Plants: Principles and Perspectives. pp. 29–49. John Wiley and Sons, New York.
Esau, K. 1965. Plant Anatomy, 2 ed. John Wiley and Sons, New York.
Green, P.B. 1980. Organogenesis—a biophysical view. Annual Rev. Plant Physiol.31: 51–82.
—. 1985. Surface of the shoot apex: a reinforcement-field theory for phyllotaxis. J. Cell Sci. Suppl.2: 181–201.
— andK.E. Brooks. 1978. Stem formation from a succulent leaf: its bearing on theories of axiation. Amer. J. Bot.65: 13–26.
— andJ.M. Lang. 1981. Toward a biophysical theory of organogenesis: birefringence observations on regenerating leaves in the succulent,Graptopetalum paraguayense E. Walther. Planta151: 413–426.
Gunckel, J.E. andK.V. Thimann. 1949. Studies of development in long shoots and short shoots ofGinkgo biloba L. III. Auxin production in shoot growth. Amer. J. Bot.36: 145–151.
— andR.H. Wetmore. 1946. Studies of development in long shoots and short shoots ofGinkgo biloba L. I. The origin and pattern of development of the cortex, pith and procambium. Amer. J. Bot.33: 285–295.
Gunning, B.E.S. andA.R. Hardman. 1982. Microtubules. Annual Rev. Plant Physiol.33: 651–698.
Hara, N. 1971. Structure of the vegetative shoot apex ofClethra barbinervis III. Longisectional view, summary analysis and discussion. Bot. Mag. Tokyo84: 283–292.
Hardham, A.R., P.B. Green andJ.M. Lang. 1980. Reorganization of cortical microtubules and cellulose deposition during leaf formation inGraptopetalum paraquayense. Planta149: 181–195.
Iwata, K. andT. Hogetsu. 1989. The effects of light irradiation on the orientation of microtubules in seedlings ofAvena sativa L. andPisum sativum L. Plant Cell Physiol.30: 1011–1016.
Jesuthasan, S. andP.B. Green. 1989. On the mechanism of decussate phyllotaxis: Biophysical studies on the tunica layer ofVinca major. Amer. J. Bot.76: 1152–1166.
Lang Selker, J.M. andP.B. Green. 1984. Organogenesis inGraptopetalum paraquayense E. Walther: shifts in orientation of cortical microtubule arrays are associated with periclinal divisions. Planta160: 289–297.
Lintilhac, P.M. andP.B. Green. 1976. Patterns of microfibrillar order in a dormant fern apex. Amer. J. Bot.63: 726–728.
Marc, J. andW.P. Hackett. 1989. A new method for immunofluorescent localization of microtubules in surface cell layers: application to the shoot apical meristem ofHedera. Protoplasma148: 70–79.
Mita, T. andM. Katsumi. 1986. Gibberellin control of microtubule arrangement in the mesocotyl epidermal cells of the d5 mutant ofZea mays L. Plant Cell Physiol.27: 651–659.
— andH. Shibaoka. 1874. Gibberellin stabilizes microtubules in onion leaf sheath cells. Protoplasma119: 100–109.
Negbi, M., B. Baldev andA. Lang. 1964. Studies on the orientation of the mitotic spindle in the shoot apex ofHyoscyamus niger and other rosette plants. Israel J. Bot.13: 134–153.
Peachey, L.D. 1958. Thin sections I. A study of section thickness and physical distortion produced during microtomy. J. Biophys. Biochem. Cytol.4: 233–242.
Roberts, I.N., C.W. Lloyd andK. Roberts. 1985. Ethylene-induced microtubule reorientations: mediation by helical arrays. Planta164: 439–447.
Romberger, J.A. 1963. Meristems, growth, and development in woody plants. An analytical review of anatomical, physiological, and morphogenic aspects. U.S. Dept. of Agriculture, Technical Bull. No. 1293.
Rouffa, A.S. andJ.E. Gunckel. 1951. Leaf initiation, origin, and pattern of pith development in the Rosaceae. Amer. J. Bot.38: 301–307.
Sakaguchi, S, T. Hogetsu andN. Hara. 1988a. Arrangement of cortical microtubules in the shoot apex ofVinca major L. Observations by immunofluorescence microscopy. Planta175: 403–411.
. 1988b. Arrangement of cortical microtubules at the surface of the shoot apex inVinca major L.: Observations by immunofluorescence microscopy. Bot. Mag. Tokyo101: 497–507.
Shibaoka, H. 1974. Involvement of wall microtubules in gibberellin promotion and kinetin inhibition of stem elongation. Plant Cell Physiol.15: 255–263.
Spurr, A.R. 1969. A low-viscosity epoxy resin embedding medium for electron microsopy. J. Ultrastruct. Res.26: 31–43.
Titman, P.W. andR.H. Wetmore. 1955. The growth of long and short shoots inCercidiphyllum. Amer. J. Bot.42: 364–372.
Tolbert, R.J. 1961. A seasonal study of the vegetative shoot apex and the pattern of pith development inHibiscus syriacus. Amer. J. Bot.48: 249–255.
Venable, J.H. andR.A. Coggeshall. 1965. A simplified lead citrate stain for use in electron microscopy. J. Cell Biol.25: 442–445.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sakaguchi, S., Hogetsu, T. & Hara, N. Specific arrangements of cortical microtubules are correlated with the architecture ofmeristems in shoot apices of angiosperms and gymnosperms. Bot Mag Tokyo 103, 143–163 (1990). https://doi.org/10.1007/BF02489622
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02489622