Abstract
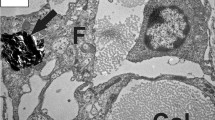
Changes in the nerve fibers of the spinal cord were studied in rat experimental epidural tumor models. Light microscopy showed demyelinization in all with rats paraparesis and paraplegia. Cross-sectional views of nerve fibers stained with 3,3dipentyloxacarbo-cyanine iodide, obtained by confocal laser scanning microscopy, showed distorted, shrunken fibers with a low fluorescence intensity. Changes in the electrolyte contents of nerve fibers were studied by electron probe X-ray microanalysis. The K concentration in axons and the myelin sheath was increased in the paraparesis group, but was decreased in the paraplegia group. These findings suggest that, in the paraparesis group, compression of the spinal cord damaged cell membrane channels, which subsequently caused an increase in intracellular K, a decline in the action potential, and low-intensity fluorescence of nerve fibers. On the other hand, in the paraplegia group, destruction of cell membranes caused a decrease in intracellular K until it approached the extracellular level. This reduced both the action potential and the fluorescence intensity. As Ca and Mg concentrations in both axons and the myelin sheath increased in relation to the severity of neurologic damage, it appears that these electrolytes may also play an important role in damage to nerve fibers.
Similar content being viewed by others
References
Aoki Y, Tsuji H, Maruo S, et al. Electrolyte measurements by electron probe X-ray microanalysis in acute spinal cord compression model (in Japanese). Central Jpn J Orthop Surg Traumatol 1994;37:385–6.
Arbit E, Galicich W, Galicich JH, et al. An animal model of epidural compression of the spinal cord. Neurosurgery 1989;24:860–3.
Balentine JD. Pathology of experimental spinal cord trauma I. Necrotic lesion as a function of vascular injury. Lab Invest 1978;39:236–53.
Balentine JD. Pathology of experimental spinal cord trauma II. Ultrastructure of axons in myelin. Lab Invest 1978;9:254–66.
Barron KD, Hirano A, Araki S, et al. Experiences with metastatic neoplasms involving the spinal cord. Neurology 1959;9:91–106.
Batson OV. The function of the vertebral veins and their role in the spread of metastases. Ann Surg 1940;112:138–49.
Bennett MH, McCallum JE. Experimental decompression of spinal cord. Surg Neurol 1977;8:63–7.
Blakemore WF. Observations on remyelination in the rabbit spinal cord following demyelination induced by lysolecithin. Neuropathol Appl Neurobiol 1978;4:45–59.
Blaustein MP, Goldring JM. Membrane potentials in pinched-off presynaptic nerve terminals monitored with a fluorescent probe: Evidence that synaptosomes have potassium diffusion potentials. J Physiol 1975;247:589–615.
Boo DS, Tsuji H, Maruo S, et al. A study of compressed spinal cord by confocal laser scanning microscopy using potential sensitive molecular probes in membranes (in Japanese). Central Jpn J Orthop Surg Traumatol 1994;37:1209–10.
Cohen LB, Salzberg BM, Davila HV, et al. Changes in axon fluorescence during activity: Molecular probes of membrane potential. J Membrane Biol 1974;19:1–36.
Coman DR, deLong RP. The role of the vertebral venous system in the metastasis of cancer to the spinal column. Cancer 1951:610–8.
Delattre J-Y, Arbit E, Rosenblum MK, et al. High dose versus low dose dexamethasone in experimental epidural spinal cord compression. Neurosurgery 1988;22:1005–7.
Gupta BL, Hall TA. Electron microprobe X-ray analysis of calcium. Ann New York Acad Sci 1978;307:28–51.
Hukuda S, Wilson CB. Experimental cervical myelopathy: Effects of compression and ischemia on the canine cervical cords. J Neurosurg 1972;37:631–52.
Izumida M. A chronic spinal cord compression model in a rat with a 354A tumor (in Japanese). J Jpn Orthop Assoc 1995;69:977–91.
Kamino K, Hirota A, Fujii S. Experimental background of optical measurement of membrane potential (in Japanese). Membrane 1981;6:93–103.
Kobayashi N. An experimental study on the effect of dorsal compression to injured spinal cord using spinal cord evoked potentials (in Japanese). Central Jpn J Orthop Surg Traumatol 1995;38:549–60.
Lee C-S. The changes of the spinal evoked potential caused by constant-speed subacute compression of the spinal cord. J Jpn Orthop Assoc 1985;59:949–60.
Nakagaki I, Sasaki S, Shiguma M, et al. Distribution of elements in pancreatic exocrine cells determined by electron probe X-ray microanalysis. Pflügers Arch 1984;401:340–5.
Rubin P. Extradural spinal cord compression by tumor. Radiology 1969;93:1243–60.
Sakou T, Tomimura K, Maebara T, et al. Acute and chronic compression on the spinal cord and the response on the permeability of the microvasculature. An experimental study (in Japanese). J Jpn Orthop Assoc 1977;51:215–23.
Sasaki S, Nakagaki I, Mori H, et al. Intracellular calcium store and transport of elements in acinar cells of the salivary gland determined by electron probe X-ray microanalysis. Jpn J Physiol 1983;33:69–83.
Sasaki S, Sawada Y, Morita M, et al. Effects of benzothiazepine calcium blocker on electrolyte alteration in human ischemic and reperfused myocardium. J Mol Cell Cardiol 1994;26:1037–44.
Schramm J, Takahashi H, Krause R. Reversibility of changes in evoked spinal response following graded spinal cord compression. Acta Neurochir (Wien) 1979;28:599–604.
Schramm J, Shigeno T, Brock M. Clinical signs and evoked response alterations associated with chronic experimental cord compression. J Neurosurg 1983;58:734–41.
Shibahara N, Okada S, Onishi S, et al. Axoplasmic electrolyte contents measured by X-ray microanalysis in experimental uremic neuropathy. Jpn J Nephrol 1990;32:37–44.
Talrov IM, Klinger H, Vitale S. Spinal cord compression studies. I. Experimental techniques to produce acute and gradual compression. Arch Neurol Psychiat 1953;70:813–9.
Ushio Y, Posner R, Posner JB, et al. Experimental spinal cord compression by epidural neoplasms. Neurology 1977;27:422–9.
Young W. The role of calcium in spinal cord injyry. CNS Trauma 1985;2:109–14.
Author information
Authors and Affiliations
About this article
Cite this article
Aoki, Y., Maruo, S., Arakawa, A. et al. Experimental study of spinal cord compression by epidural tumors using electron probe X-ray microanalysis and confocal laser scanning microscopy. J Orthop Sci 2, 434–441 (1997). https://doi.org/10.1007/BF02488932
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02488932