Summary
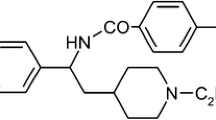
SD3212 is a new antiarrhythmic drug which has class I, III, and IV effects. The purpose of this study was to elucidate the electrophysiological effects of this compound on a rabbit atrial fibrillation model, and to test a hypothesis that atrial fibrillation threshold is a quantitative indicator of atrial vulnerability. Whole hearts were excised from rabbits, and the aortas cannulated to perfuse the coronary arteries. Atrial fibrillation was induced with a burst stimulation of 50 Hz for 1 s while 3µM acetylcholine (ACh) was perfused. When the right atrial appendage was paced at 200-ms intervals, SD3212 prolonged interatrial conduction time: control 30 ± 1.2ms, ACh 33 ± 1.4ms, ACh + SD 1µM 37 ± 2.4ms, ACh + SD 3µM 52 ± 8.1ms. The drug also prolonged the effective refractory period: control 80 ± 3.0ms, ACh 48 ± 3.8ms, ACh + SD 1µM 65 ± 4.7ms, ACh + SD 3 µM 98 ± 15ms. The rate of induction of atrial fibrillation by rapid pacing was 26% in Tyrode's solution, 85% in the presence of ACh, and 38% in the presence of ACh + SD 1 µM. The atrial fibrillation threshold decreased from 8.6 ± 0.8 mA (control) to 2.5 ± 0.7 mA in the presence of ACh. It increased again to 7.8 ± 1.0 mA in the presence of SD3212 (1 µM). SD3212 prolonged both the conduction time and refractory period. A reversed use-dependency was not prominent. These features caused antifibrillatory effects. Thus, the atrial fibrillation threshold seems to be a good quantitative indicator of atrial vulnerability.
Similar content being viewed by others
References
Natale A, Blanck Z, Deshpande S, Dhala A, Jazayeri M, Sra J, Akhtar M (1995) Current concerns and future directions in the pharmacological treatment of atrial fibrillation. In: DiMarco JP, Prystowsky EN (eds) Atrial arrhythmias. State of the art. Futura, New York, pp 387–401
Gilligan DM, Ellenbogen KA, Epstein AE (1996) The management of atrial fibrillation. Am J Med 101:413–421
Ganz LI, Antman EM (1997) Antiarrhythmic drug therapy in the management of atrial fibrillation. J Cardiovasc Electrophysiol 8:1175–1189
Singh BN (1996) Antiarrhythmic actions of amiodarone: a profile of a paradoxical agent. Am J Cardiol 78(4A):41–53
Maisel WH, Kuntz KM, Reimold SC, Lee TH, Antman EM, Friedman PL, Stevenson WG (1997) Risk of initiating antiarrhythmic drug therapy for atrial fibrillation patients admitted to a university hospital. Ann Intern Med 127:281–284
Grey E, Silverman DI (1993) Efficacy of type IC antiarrhythmic agents for treatment of resistant atrial fibrillation. PACE 16:2235–2240
Herre JM, Sauve MJ, Malone P, Griffin JC, Helmy I, Langberg JJ, Goldberg H, Scheinman MM (1989) Longterm results of amiodarone therapy in patients with recurrent sustained ventricular tachycardia or ventricular fibrillation. J Am Coll Cardiol 13:442–449
Echt DS, Liebson PR, Mitchell LB, Peters RN, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL, Huther ML, Richardson DN, CAST investigators (1991) Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med 324:781–788
Kodama I, Suzuki R, Maruyama K, Toyama J (1995) Electrophysiological effects of SD-3212, a new antiarrhythmic agent with vasodilator action, on guinea-pig ventricular cells. Br J Pharmacol 114:503–509
Takahashi N, Ito M, Ishida S, Fujino T, Maruyama T, Saikawa T (1995) Electrophysiological effects of SD-3212, a novel antiarrhythmic agent, on rabbit hearts in vivo and in vitro. J Cardiovasc Pharmacol 25:1006–1011
Hondeghem LM, Snyders DJ (1990) Class III antiarrhythmic agents have a lot of potential but a long way to go. Reduced effectiveness and danger of reverse usedependence. Circulation 81:686–690
Hara Y, Nakaya H (1995) SD-3212, a new class I and IV antiarrhythmic drug: a potent inhibitor of the muscarinic acetylcholine receptor-operated potassium current in guinea-pig atrial cells. Br J Pharmacol 116:2750–2756
Nagashima S, Uematsu T, Araki S, Matsuzaki T, Fukuchi M, Hashimoto H, Nakashima M (1992) Antiarrhythmic and electrophysiological effects of SD-3212, a novel Na+ and Ca++ channel blocker, in anaesthetized dogs with myocardial infarction in comparison with its stereoisomer (SD-3212) and bepridil. Naunyn-Schmiedeberg's Arch Pharmacol 345:688–695
Nakayama K, Morimoto K, Nozawa Y, Tanaka Y (1992) Calcium antagonistic and binding properties of semotiadil (SD-3211), a benzothiazepine derivative assessed in cerebral and coronary arteries. J Cardiovasc Pharmacol 20:380–391
Fukuchi M, Uematsu T, Nagashima S, Nakashima M (1990) Antiarrhythmic effects of a benzothiazepine derivative (SD-3211) and its stereoisomer (SD-3212) in anaesthetized rats and islated perfused rat hearts compared with bepridil. Naunyn-Schmiedeberg's Arch Pharmacol 341:557–564
Miyawaki N, Yamazaki F, Furuta T, Shigei T, Yamauchi H (1991) Antiarrhythmic effects of a novel Na+ and Ca2+ channel blocker SD-3212: a comparison with its enantiomer (SD-3211). Drug Dev Res 22:293–298
Hirasawa A, Haruno A, Matsuzaki T, Hashimoto K (1992) Effects of new antiarrhythmic drug, SD-3212, on canine ventricular arrhythmia models. Jpn Heart J 33:851–861
Allessie MA, Lammers WJEP, Bonke IM, Hollen J (1984) Intra-atrial reentry as a mechanism for atrial flutter induced by acetylcholine and rapid pacing in the dog. Circulation 70:123–135
Euler DE, Scanlon PJ (1987) Acetylcholine release by a stimulus train lowers atrial fibrillation threshold. Am J Physiol 253:H863-H868
Inoue M, Inoue D, Ishibashi K, Sakai R, Omori I, Yamahara Y, Asayama J, Nakagawa M (1993) Effects of pilsicainide on the atrial fibrillation threshold in guinea pig atria. A comparative study with disopyramide, lidocaine and flecainide. Jpn Heart J 34:301–312
Ravelli F, Allessie MA (1997) Effects of atrial dilatation on refractory period and vulnerability to atrial fibrillation in the isolated Langendorff-perfused rabbit heart. Circulation 96:1686–1695
Ishibashi K, Inoue D, Sakai R, Inoue M, Shirayama T, Asayama J, Nakagawa M (1995) Effects of disopyramide on the atrial fibrillation threshold in the human atrium. Int J Cardiol 52:177–184
Wijffel MCE, Kirchhof CJHJ, Dorland R, Allessie MA (1995) Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 92:1954–1968
Rensma PL, Allessie MA, Lammers WJ, Bonke FI, Schalij MJ (1988) Length of excitation wave and susceptibility to reentrant atrial arrhythmias in normal conscious dogs. Circ Res 62:395–410
Toda I, Murakami Y, Nozaki A, Kawakubo K, Sugimoto T (1988) Ventricular fibrillation threshold measured by continuous 50 cps stimulation for the evaluation of the antifibrillatory effect of the drugs. Jap Circ J 52:249–253
Sugimoto T, Schaal SF, Wallace AG (1967) Factors determining vulnerability to ventricular fibrillation induced by 60-cps alternating current. Circ Res 11:601–608
Coumel P (1992) Neural aspects of paroxysmal atrial fibrillation. In: Falk RH, Podrid PJ (eds) Atrial fibrillation: mechanisms and management. Raven, New York, pp 109–125
Janse MJ (1989) Anisotropic conduction in the atrium: its role in arrhythmogenesis. In: Attuel P, Coumel P (eds) The atrium in health and disease. Futura, New York, pp 15–26
Schuessler RB, Boineau JP, Bromberg BI, Hand DE, Yamauchi S, Cox JL (1995) Normal and abnormal activation of the atrium. In: Zipes DP, Jalife J (eds) Cardiac electrophysiology from cell to bedside. Saunders, Philadelphia, pp 543–562
Spach MS (1995) Nonuniform anisotropic cellular coupling as a basis for reentrant arrhythmias. In: DiMarco JP, Prystowsky EN (eds) Atrial arrhythmias. State of the art. Futura, New York, pp 123–147
Papageorgiou P, Monahan K, Boyle NG, Seifert MJ, Beswick P, Zebede J, Epstein LM, Josephson ME (1996) Site-dependent intra-atrial conduction delay. Relationship to initiation of atrial fibrillation. Circulation 94:384–389
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Matsuo, R., Shirayama, T., Inoue, K. et al. SD3212, a new antiarrhythmic drug, raises atrial fibrillation threshold in isolated rabbit hearts. Heart Vessels 14, 127–136 (1999). https://doi.org/10.1007/BF02482296
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02482296