Abstract
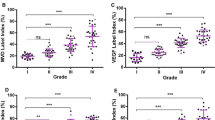
The matrix metalloproteinase (MMP) family members catalyze extracellular proteolysis. Recent reports have suggested that expression of MMP-2 and-9 might play a critical role in neoplastic tissue invasion or metastasis. In this study, the relationship between the expression of MMP-2 and-9 and the histological features of tissues from 21 cases of human glioma were investigated. MMP-2 and-9 proteins were detected by immnohistochemical studies. Amplification of MMP-2 and-9 mRNA was detected by reverse transcription-polymerase chain reaction (RT-PCR) assay. MMP-2 and-9 mRNA was measured quantitatively by the real-time RT-PCR method. Immunohistochemically, 38% of the cases were positive for MMP-2. Amplification of MMP-2 mRNA by RT-PCR was detected in. 62% of the cases. There was no significant relationship between the expression of MMP-2 protein or mRNA and the biological nature of the tumors, including aggressiveness and histologic classification. The quantity of MMP-2 mRNA was 0.035±0.113 (MMP-2/GAPDH%), which was significantly elevated in cases of neoplastic dissemination or recurrence (P<0.05). Tumor cells were immunohistochemically positive for MMP-9 in 81% of the samples. A positive reaction was found not only in neoplastic cells but also in endothelial cells, suggesting that the expression of MMP-9 protein might be associated with tumoral angiogenesis. The expression of mRNA in MMP-9 was detected in 91% of the cases, suggesting a close relationship between expression of MMP-9 and malignancy. The quantity of MMP-9 was 0.097±0.113 (MMP-9/GAPDH %) in all samples, which was significantly elevated in cases of glioblastoma (P<0.05). The average Ki-67 labeling index was 8.14±5.26 in samples from G2 glioma, 19.92±11.29 in samples from G3 glioma, and 23.52±10.14 in samples from glioblastoma. All of the cases with elevated indices had recurrence or dissemination. The results of our study suggest that quantity analyses of MMP-2 and-9 mRNA and Ki-67 labeling index should be useful for discerning tumoral behaviors such as invasion, dissemination, and recurrence.
Similar content being viewed by others
References
Muller D, Quantin B, Gesnel MC, et al (1988) The collagenase family in humans consists of at least four members. Biochem J 253:187–192
Belaaouaj A, Shipley JM, Kobayashi DK, et al (1993) Human macrophage metalloelastase: genomic organization, chromosomal location, gene linkage, and tissue-specific expression. J Biol Chem 270:14568–14575
Scott MW, Ivan EC, Barry LM, et al (1989) SV40-transformed human lung fibroblasts secrete a 92-kDa type IV collagenase which is identical to that secreted by normal human macrophages. J Biol Chem 64:17213–17221
Mon-LI C, Dorothy C, Te-cheng P, et al (1988) Amino acid sequence of the triple-helical domain of human collagen type IV. J Biol Chem 263:18601–18606
Collier IE, Brus G, Goldberg GI, et al (1991) On the structure and chromosome location of the 72-and 92-kDa human type IV collagenase genes. Genomics 9:429–434
Pirkko H, Ari T, Louise TC, et al (1991) Complete structure of the human gene for 92-kDa type IV collagenase. J Biol Chem 266:16485–16490
Sawaya RE, Yamamoto M, Gokaslan ZL, et al (1996) Expression and localization of 72kDa type IV collagenase (MMP-2) in human malignant gliomas in vivo. Clin Exp Metastasis 14:35–42
Timothy E, Van M, Harcharan K, et al (2001) The role of matrix metalloproteinase genes in glioma invasion: co-dependent and interactive proteolysis. J Neurooncol 53:213–235
Hajime Y, Ken-ichi M, Masayuki T, et al (2000) Expression and tissue localization of membrane-types 1, 2 and 3 matrix metalloproteinase in rheumatoid synovium. Lab Invest 80:677–687
Stanley Z, Rita ML, Mohammad H, et al (1993) 92000 type IV collagenase is increased in plasma of patients with colon cancer and breast cancer. Cancer Res 53:140–146
Davies B, Waxman J, Wasan H, et al (1993) Levels of matrix metalloproteinases in bladder cancer correlate with tumor grade and invasion. Cancer Res 53:5365–5369
Porsyth PA, Laing TD, Gibson AW, et al (1998) High levels of gelatinase B and active gelatinase in metastatic glioblastoma. J Neurooncol 36:21–29
Sato H, Takino T, Okada Y, et al (1994) A matrix metallo-proteinase expressed on the surface of invasive tumor cells. Nature 370:61–65
Coussens LM, Ingleton B, Matrisian LM (2002) Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 295:2387–2392
Yan L, Moses MA, Huang S, et al (2000) Adhesion-dependent control of matrix metalloproteinase-2 activation in human capillary endothelial cells. J Cell Sci 113:3979–3987
Ivan EC, Scott MW, Arthur ZE, et al (1988) II-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem 263:6579–6587
Liotta LA, Steeg PS, Stetler-Stevenson WG (1991) Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 64:327–336
Ernst L, Barbara S, Elisabeth K, et al (2001) Expression of latent matrix metalloproteinase 9 (MMP-9) predicts survival in advanced ovarian cancer. Gynecol Oncol 82:291–298
Maode W, Tuo W, Shuoxun L, et al (2003) The expression of matrix metalloproteinase-2 and-9 in human gliomas of different pathological grades. Brain Tumor Pathol 20:65–72
Yoshida D, Piepmeir JM, Bergenheim T, et al (1998) Suppression of metalloproteinase-2 and mediated cell invasion in U87MG, human glioma cells by anti-microtubule agent in vitro study. Br J Cancer 77:21–25
Kondraganti S, Mohanam S, Chintala SK, et al (2000) Selective suppression of matrix metalloproteinase-9 in glioblastoma cells by antisense gene transfer impairs glioblastoma cell invasion. Cancer Res 60:6851–6855
Bergers G, Brekken R, McMahon G, et al (2000) Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2:737–744
Yang-Feng TL, Berliner N, Deverajan P, et al (1991) Assignment of two human neutrophil collagenases to chromosome II. Cytogenet Cell Genet 58:1974
Tetsuo S, Kazuki N, Bryan PT, et al (2000) Glioma cell extracellular matrix metalloproteinase inducer (EMMPRI N)(CD147) stimulates production of membrane-type co-cultures with brain-derived fibroblasts. Cancer Lett 157:177–184
Azzaq BJ, Michael S, Dale KK, et al (1995) Human macrophage metalloelastase. J Biol Chem 270:14568–14575
Elena ID, Mario AB, Ralph AR, et al (1998) Remodeling of collagen matrix by human tumor cells requires activation and cell surface association of matrix metalloproteinase-2. Cancer Res 58:3743–3750
Mitsutoshi N, Hiroyuki N, Eiji I, et al (1999) Expression and tissue localization of membrane-type 1, 2, and 3 matrix metalloproteinases in human astrocytic tumors. Am J Pathol 154:417–428
Marbaix E, Donnez J, Courtoy PJ, et al (1992) Progesterone regulates the activity of collagenase and related gelatinases A and B in human endometrial explants. Proc Natl Acad Sci USA 89:11789–11793
Collier IE, Bruns G, Goldberg GI, et al (1991) On the structure and chromosome location of the 72-and 92-kDa human type IV collagenase genes. Genomics 9:429–434
Deryugina EI, Bourdon MA, Reisfeld RA et al (1998) Remodeling of collagen matrix by human tumor cells requires activation and cell surface association of metalloproteinase-2. Cancer Res 58:3743–3750
Axel P, Eva MW, Ghislain O, et al (2001) Distinct expression patterns and levels of enzymatic activity of matrix metallo-proteinases and their inhibitors in primary brain tumors. J Neuropathol Exp Neurol 60:598–612
Duc ML, Arnaud B, Darrin K, et al (2003) Exploitation of astrocytes by glioma cells to facilitate invasiveness: a mechanism involving matrix metalloproteinase-2 and the urokinase-type plasminogen activator-plasmin cascade. J Neurosci 23:4034–4043
Cornelis FMS, Giovanni C, Jan HV, et al (2000) Enhanced urinary gelatinase activities (matrix metalloproteinases 2 and 9) are associated with early-stage bladder carcinoma. Clin Cancer Res 6:2333–2340
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Komatsu, K., Nakanishi, Y., Nemoto, N. et al. Expression and quantitative analysis of matrix metalloproteinase-2 and-9 in human gliomas. Brain Tumor Pathol 21, 105–112 (2004). https://doi.org/10.1007/BF02482184
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02482184