Abstract
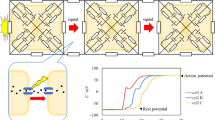
The channel gating process of neural cells is the first step of neural information transmission. We have proposed a kinetic model for state transitions for a sodium (Na) ion gating channel under H2 control. The channel state consisted of an open state, three closed but activated states, and four inactivated but not closed states. This modeling was based strictly on molecular biological observations. Three charged amino acid helixes of the specific subunits of the Na channel hole act as activating gates. Another helix of the subunit having membrane voltagesensing properties behaves as an inactivating particle that invades the Na channel hole after membrane depolarization. This particle blocks the free movements of the three activating gates and inactivates the Na channel gating function. In total there are eight channel states, which consist of four inactivated states, three closed states, and one open state. We expressed the transitions among these states by eight linear differential equations using the law of conservation. For the control principle, the channel system is always exposed to a biological mimetic that is a false transmitter and competes for the channel sites with Na ions. Hence, we regarded such biological agencies as noises in the system that disturb the effective transmission of information, i.e., rapid transitions through the channel gating systems. The physiological Na gating is understood to minimize influences from the disturbing noises on the transition of the channels states, and we have proposed the H2 control principle. The computed results of temporal changes in the amount of channel species per unit membrane area showed rapid changes and then termination. This behavior was strongly dependent on the membrane potential. Our modeling could describe the rapid excitation and resetting of the Na ion channel gating function of the neural system. These results strongly reflect the digital nature of the neural system. The present investigation could be used to evaluate the function of neural systems that minimizes the influences of noises on the information transmission process by the transitions of the Na ion channel gating state.
Similar content being viewed by others
References
Stryer L (1991) Biochemistry, 3rd ed. Freeman, New York
Hodgkin AL, Huxley AF (1952) Quantitative description of membrane current and its application to conductance and excitation in nerve. J Physiol 117:500–544
Vandenberg CA, Bezanilla F (1991) A sodium channel gating model based on single channel macroscopic ionic and gating currents in the squid giant axon. Biophys J 60:1511–1533
Patlak J (1991) Activation kinetics of sodium channels. Physiol Rev 71:1047–1080
Armstrong CM (1981) Sodium channels and gating currents. Physiol Rev 61:644–683
Armstrong CM, Bezanilla F (1977) Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol 70:567–590
Hirayama H (1998) Optimal control of active transport across a biological membrane. Artif Life Robotics 2:33–40
Zhou KM, Doyle JC (1998) Essentials of robust control, Prentice Hall, Englewood Cliffs
Noda M, Ikeda T, Kayano T (1987) Primary structure of the electrophoresis sodium channel deduced from the cDNA sequence. Nature London 312:121–127
Guy H (1990) Models of voltage and transmitter activated membrane channel. In: Pasternark C (ed) Monovalent cations in biological systems. CRC 19909:31–58
Benndorf K (1989) A reinterpretation of Na channel gating. Eur Biophys J 17:257–271
Krafte D, Goldin AL, Auld VJ, et al. (1990) Inactivation of cloned Na channels expressed inXenopus oocytes. J Gen Physiol 96:689–706
Sheets MF, Hanck DA (1995) Voltage-dependent open-state inactivation of cardiac sodium channels. J Gen Physiol 106:617–640
Keynes RD (1991) On the voltage dependence of inactivation in the sodium channel of the squid gigant axon. Proc R Soc B 243:47–53
Horn R, Patlak J, Stevens CF (1981) Sodium channels need not open before they inactivate. Nature 291:426–427
Patlak J, Horn R (1982) The effect of N-bromacetamide on single sodium-channel currents in excised membrane patches. J Gen Physiol 79:333–351
Meves H, Vogel W (1978) Inactivation of the sodium permeability in squid giant nerve fibers. Prog Biophys Mol Biol 33:207–230
French RJ, Horn R (1983) Sodium channel gating models, mimics and modifiers. Annu Rev Biophys Bioeng 12:319–356
O'Leart ME, Chen Li-Q, Kallen RG, et al. (1995) A molecular link between activation and inactivation of the sodium channel. J Gen Physiol 106:641–658
Horn R, Venderberg CA (1984) Statistical properties of single sodium channels. J Gen Physiol 84:505–534
Nonner W (1980) Relations between the inactivation of sodium channels and the immobilization of the gating charge in frog myelinated nerve. J Physiol 299:573–603
Hill B (1992) Ionic channels of excitable membranes, 2nd edn. Sinauer, Sunderland
Stimers JR, Bezanilla F, Taylor RE (1985) Sodium channel activation in the squid giant axon. Steady state properties. J Gen Physiol 85:65–82
Dubois JM, Schneider MF (1982) Kinetics of intramembrane charge movement and sodium current in the frog node of ranvier. J Gen Physiol 79:571–602
Oxford GS (1981) Some kinetic and steady state properties of the sodium channel after removal of inactivation. J Gen Physiol 77:1–22
Keynes RD (1985) Modeling the sodium channel. In: Rotchie J, Keynes RE, Bolis L (eds) Ion channels in neural membranes. Proceedings of the 11th International Conference on Biological Membranes. Alan R Liss, New York, p 86–101
Patlak J, Horn R (1982) The effect of N-bromoacetamide on single sodium channel currents in excised membrane patches. J Gen Physiol 79:333–351
Catterall WA (1986) Voltage dependent gating of sodium channels. Correlating structure and functions. Trends Neurosci 9:7–10
Zhou K, Doyle JC (1998) Essentials of Robust Control. Prentice Hall, Englewood Cliffs
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hirayama, H., Okita, Y. H2 control strategy for Na ion channels on the neural cellular membrane. Artif Life Robotics 5, 120–131 (2001). https://doi.org/10.1007/BF02481349
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02481349