Abstract
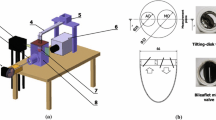
An experimental investigation was conducted to determine the velocity fields, magnitudes of shear stresses, and regions of stagnation of a jellyfish valve and a St. Vincent tilting-disk valve using laser Doppler anemometry (LDA). All experiments were performed in vitro and at steady volumetric flow rates of 15 and 261/min (representing peak systole). The St. Vincent valve flow field was very unsymmetrical in the measurement plane that spanned the major and minor outflow regions of the valve and persisted at least to 1D downstream. For both flow rates tested, a stagnation region was always observed just behind the occluder. The maximum axial velocity was in the major outflow side and reached 2.54 m/s for peak systole flow of 261/min. Moreover, in the immediate vicinity of the valve and for both flow rates tested, turbulence intensities and velocity gradients were higher in the minor outflow region than in the major outflow region. However, as the flow progressed downstream, the opposite was observed, with large peak velocity in the major outflow. The maximum shear stress across the St. Vincent valve occurred in the minor outflow region and increased from 30 to 60N/m2 as the flow rate increased from 15 to 261/min. In the core of the larger jet, the shear stresses were very small (0–3N/m2). The flow at the edges of the jellyfish valve membrane, 10 mm (0.5D) downstream from the ring valve, consisted of two nearly symmetric jets in the vicinity of the tube wall. The maximum axial velocity in these jet regions increased from 1.7 to 2.5 m/s as the flow rates increased from 15 to 261/min. Pressure effects due to the oscillation of the membrane of the jellyfish valve appear to generate high shear stress in the immediate vicinity of the jellyfish valve (0.5D downstream). The values of shear stress were 0–27 N/m2 for a flow of 151/min and 3–109 N/m2 for a flow of 261/min. However, as the flow progresses downstream, shear stresses decay rapidly and return to the upstream undisturbed level at about 4D downstream, but at a slower rate than the RMS axial velocities. In general, for all operating flow conditions tested here, the jellyfish valve performed better than the St. Vincent valve when velocity and shear stress distributions are compared at locations more than 0.5D downstream from the valve.
Similar content being viewed by others
References
Hufnagel CA, Harvey WP, Rabil PJ, McDermott TF. Surgical corection of aortic insufficiency. Surgery 1954;35:673–683
Starr A, Edwards ML, McCord CW, Griswold HH. Aortic replacement clinical experience with semirigid ball-valve prosthesis. Circulation 1963;29:779–783
Yoganathan AP, Corcoran WH, Harrsion EC, Carl JR. In vitro velocity measurements in the near vicinity of the Bjork-Shiley aortic prosthesis using LDA. Med Biol Eng Comp 1979;17:453–459
Stein PD, Sabbah HN. Fluid stresses, platelets and bioprosthetic valves. Proc 37th Annual Conference on Engineering in Medicine and Biology. Los Angeles, Calif, 1984;144
Tillmann W, Reul H, Herold M, Bruss KH, van Gilse J. In-vitro wall shear measurements at aortic valve prostheses. J Biomech 1984;17:263–279
Hanle DD, Harrison EC, Yoganathan AP, Corcoran WH. Turbulence downstream from the Ionescu-Shiley bioprosthesis in steady and pulsatile flow. Med Biol Eng Comput 1987;25:645–649
Tiederman WG, Steinle MJ. Two component laser velocimeter measurements downstream of heart valve prostheses in pulsatile flow. J Biomech Eng 1986;108:59–64
Yoganathan AP, Woo YR, Sung HW. Turbulent sheart stress measurements in the vicinity of aortic heart valve prostheses. J Biomech 1986;19:433–442
Hanle DD, Harrison EC, Yoganathan AP, Allen DT, Corcoran WH. In vitro flow dynamics of four prosthetic aortic valves: a comparative analysis. J Biomech 1989;22:597–607
Yoganathan AP, Reamer HH, Corcoran WH, Harrison EC, Shulman IA, Parnassus W. The Starr-Edwards aortic ball valve: flow characteristics, thrombus formation, and tissue overgrowth. Artif Organs 1981;5:6–17
Hasenkam JM, Nygaard H, Giersiepen M, Reul H, Stodkilde-Jorgensen H. Turbulent stress measurements downstream of six mechanical aortic valves in a pulsatile flow model. J Biomech 1988;21:631–645
Einav S, Stolero D, Avidor JM, Elad D, Talbot L. LDA evaluation of wall shear stress distribution along the cusp of a tri-leaflet prosthetic heart valve. Proceedings of the Fourth International Symposium on the Application of Laser Anemometry to Fluid Mechanics, Lisbon, Portugal, 1988:7.21.1-3
Einav S, Reul H, Rau G, Elad D. Shear stress related blood damage along the cusp of a tri-leaflet prosthetic valve. Int J Artif Organs 1991;14:716–720
Lei M, van Steenhoven AA, van Campen DH. Experimental and numerical analyses of the steady flow field around an aortic Bjork-Shiley standard valve prosthesis. J Biomech 1992;25:213–222
Jones KC, Tansley GD, Mazumdar J. Computational fluid mechanical evaluation of steady-flow energy losses in cardiac valve prostheses. Australas Phys Eng Sci Med 1992;15:193–201
Imachi K, Mabuchi K, Chinzei T, Abe Y, Imanishi K, Yonezawa T, Maeda K, Suzukawa M, Kouno A, Ono T, Fujimasa I, Atsumi K. A newly designed jellyfish valve for an artifical heart blood pump. Trans Am Soc Artif Intern Organs 1988;34:726–728
Imachi K, Mabuchi K, Chinzei T, Abe Y, Imanishi K, Yonezawa T, Maeda K. In vitro and in vivo evaluation of a jellyfish valve for practical use. Trans Am Soc Artif Intern Organs 1989;35:298–301
Imachi K, Mabuchi K, Chinzei T, Abe Y, Imanishi K, Yonezawa T. Fabrication of a jellyfish valve for use in an artificial heart. Trans Am Soc Artif Intern Organs 1992;38:M237-M242
Gabby S, Mcqueen DM. In-vitro hydrodynamic comparison of mitral valve prosthesis at high flow rate. J Thorac Cardiovasc Surg 1976;76:771–787
Baldwin JT, Tarbell JM. Mean velocities and Reynolds stresses within regurgitant jets produced by tilting disc valves. Trans Am Soc Artif Intern Organs 1991;37:M348-M349
Fry DL, Acute vascular endothelial changes associated with increased blood velocity gradient. Circ Res 1968;22:165–197
Fry DL. Certain histological and chemical responses of the vascular interface to acutely induced mechanical stress in the aorta of dog. Circul Res 1969;24:93–108
Vaishnav RN, Patel DJ, Atabek HB, Deshpande MD, Plowman F, Vossoughi J. Determination of the local erosion stress of the canine endothelium using a jet impingement method. J Biomech Eng 1983;105:77–83
Tansley GD. Aspects of non-Newtonian flow in prosthetic heart valve studies. Automedica 1993;15:207–226
Blackshear PL Jr, Dorman FD, Steinbach JH. Some mechanical effects that can influence haemolysis. Trans Am Soc Artif Intern Organs 1965;11:112
Leverett LB, Hellums JD. Red blood cell damage by shear stress. Biophys J 1972;12:257–273
Sallam AM, Hwang NH. Human red blood cell haemolysis in a turbulent shear flow: contribution of Reynolds sheart stresses. Biorheology 1984;21:783–797
Tansley GD, Edwards RJ, Gentle CR. Role of computational fluid mechanics in the analysis of prosthetic heart valve flow. Med Biol Eng Comp 1988;26:175–185
Giersiepen M, Wurzinger LJ, Opitz R, Reul H. Estimation of shear stress-related blood damage in heart valve prostheses—in vitro comparison of 25 aortic valves. Int J Artif Organs 1990;13:300–306
Sakhaeimanesh AA, Morsi YS, Tansley GD. In vitro steady flow velocity and shear stress measurements in the vicinity of a jellyfish valve. 12th Australasian Fluid Mechanics Conference, Sydney, Australia, December 1995, vol 2; 887–890
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morsi, Y.S., Sakhaeimanesh, A. & Clayton, B.R. Measurements of steady flow velocity and turbulent stress downstream from jellyfish and St. Vincent aortic heart valves. J Artif Organs 2, 176–183 (1999). https://doi.org/10.1007/BF02480063
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02480063