Abstract
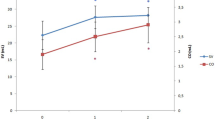
Cardiopulmonary bypass (CPB) is known to result in the abnormal production of vasoactive substances contributing to the changes in hemodynamics such as systemic vascular resistance (SVR) during and after CPB. Nitric oxide (NO) is an inflammation-mediated vasoactive substance that plays a role in the whole-body inflammatory response induced by CPB. We evaluated the role of NO in the regulation of SVR during and after CPB. Fifteen patients underwent open-heart surgery for valvular heart disease. The perfusate blood temperature of CPB was set to 34°C. The plasma levels of NO metabolites (NO −2 +NO −3 ), prostaglandin E2 (PGE2), bradykinin (BK), and systemic vascular resistance index (SVRI) were measured before CPB and 0, 12, and 24 h after CPB. The plasma level of NO metabolites increased gradually after CPB (pre-CPB, 26.3 ±4.4; 0h, 33.7±6.5; 12 h, 49.8±11.1; 24 h, 43.1±7.5 μM). SVRI decreased gradually after CPB (pre-CPB, 2361±364; 0h, 2048±216; 12 h, 1590±308; 24 h, 1727±435 dyne·s·cm−5·m2). There was a significant inverse correlation between SVRI and the plasma level of NO metabolites as a whole (r=−0.674,P<0.0001). No significant correlations were observed between SVRI and the other vasoactive substances PGE2 and BK. These findings demonstrated that NO production increased gradually during and after CPB in association with the decrease in SVR.
Similar content being viewed by others
References
Colman RW, Scott CF, Schaier AH, Wachtfogel YT, Pixley RA, Edmunds LH. Initiation of blood coagulation at artificial surfaces. Ann NY Acad Sci 1987;56:253–267
Addonizio VP. Platelet function in cardiopulmonary bypass and artificial organs. Hematol Oncol Clin North Am 1990;4:145–155
Ohata T, Sawa Y, Kadoba K, Masai T, Ichikawa H, Matsuda H. Effect of cardiopulmonary bypass under tepid temperature on inflammatory reactions. Ann Thorac Surg 1997;64:124–128
Downing SW, Edmunds LH. Release of vasoactive substances during cardiopulmonary bypass. Ann Thorac Surg 1992;54:1236–1243
Christakis GT, Fremes SE, Koch JP, Harwood S, Juhasz S, Sharpe E, Deemar KA, Hamilton C, Chen E, Rao V, Chang G, Goldman BS. Determinants of low systemic vascular resistance during cardiopulmonary bypass. Ann Thorac Surg 1994;58:1040–1049
Gordon RJ, Ravin M, Rawitscher RE, Daicoff GR. Changes in arterial pressure, viscosity and resistance during cardiopulmonary bypass. J Thorac Cardiovasc Surg 1975;69:552–561.
Myles PS, Olenikov I, Bujor MA, Davis BB. ACE-inhibitors, calcium antagonists and low systemic vascular resistance following cardiopulmonary bypass. A case-control study Med J Aust 1993; 158:675–677
Myles PS, Buckland MR, Cannon GB. The association among gastric mucosal pH, endotoxemia and low systemic vascular resistance following cardiopulmonary bypass. J Cardiothorac Vasc Anesth 1996;10:195–200
Moncada S, Radomski MW, Palmer RMJ. Endothelium-derived relaxing factor. Identification as nitric oxide and role in the control of vascular tone and platelet function. Biochem Pharmacol 1988;37:2495–2501
Faymonville ME, Deby-Dupont G, Larbuisson R. Prostaglandin E2, prostacyclin, and thromboxane changes during nonpulsatile cardiopulmonary bypass in humans. J Thorac Cardiovasc Surg 1986;91:858–866
Pang LM, Stalcup SA, Lipset JS, Hayes CJ, Bowman FO, Mellins RB. Increased circulating bradykinin during hypothermia and cardiopulmonary bypass in children. Circulation 1979;60:1503–1507
Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med 1986;315:1046–1051
Koller A, Huang A. Impaired nitric oxide-mediated flow-induced dilation in arterioles of spontaneously hypertensive rats. Circ Res 1994;74:416–421
Johnstone MT, Creager SJ, Scales KM, Cusco JA, Lee BK, Creager MA. Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. Circulation 1993;88:2510–2516
Meredith IT, Yeung AC, Weidinger FF, Anderson TJ, Uehata A, Ryan TJ Jr, Selwyn AP, Ganz P. Role of impaired endothelium-dependent vasodilation in ischemic manifestations of coronary artery disease. Circulation 1993;87(Suppl V):V56-V66
Node K, Kitakaze M, Kosaka H, Komamura K, Minamino T, Tada M, Inoue M, Hori M, Kamada T. Plasma nitric oxide end products are increased in the ischemic canine heart. Biochem Biophys Res Commun 1995;211:370–374
Node K, Kitakaze M, Kosaka H, Komamura K, Minamino T, Inoue M, Tada M, Hori M, Kamada T. Increased release of NO during ischemia reduces myocardial contractility and improves metabolic dysfunction. Circulation 1996;93:356–364
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannernbaum SR. Analysis of nitrate, nitrite, and15N nitrate in biological fluids. Anal Biochem 1982;126:131–138
Kohno M, Yasunari K, Murakawa K, Yokokawa K, Horio T, Fukui T, Takeda T. Plasma immunoreactive endothelin in essential hypertension. Am J Med 1990;88:614–618
Moncada S. The L-arginine:nitric oxide pathway. Acta Physiol Scand 1992;145:201–227
Ruvolo G, Greco E, Speziale G, Tritapepe L, Marino B, Mollace V, Nistico G. Nitric oxide formation during cardiopulmonary bypass. Ann Thorac Surg 1994;57:1055–1056
Ungureanu-Longrois D, Balligand JL, Kelly RA, Smith TW. Myocardial contractile dysfunction in the systemic inflammatory response syndrome: role of a cytokine-inducible nitric oxide synthase in cardiac myocytes. J Mol Cell Cardiol 1995;27:155–167
Ischiropoulos H, Zhu L, Beckman JS. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys 1992;298:446–451
Taylor KM, Casals JG, Mittra SM, Brannan JJ, Morton IJ. Haemodynamic effects of angiotensin converting enzyme inhibition after cardiopulmonary bypass in dogs. Cardiovasc Res 1980;14:199–205
Levine FH, Philbin DM, Kono K, Coggins CH, Emerson CW, Austen WG, Buckley MJ. Plasma vasopressin levels and urinary sodium excretion during cardiopulmonary bypass with and without pulsatile flow. Ann Thorac Surg 1981;32:63–67
Hutcheson IR, Griffith TM. Release of endothelium-derived relaxing factor is modulated both by frequency and amplitude of pulsatile flow. Am J Physiol 1991;261 (1 Pt 2):H257-H262
Sawa Y, Shimazaki Y, Kadoba K, Masai T, Fukuda H, Ohata T, Taniguchi K, Matsuda H. Attenuation of cardiopulmonary bypass derived inflammatory reactions reduces myocardial reperfusion injury in open heart surgery. J Thorac Cardiovasc Surg 1996; 111:29–35
Westaby S. Organ dysfunction after cardiopulmonary bypass. A systemic inflammatory reaction initiated by the extracorporeal circuit. Intensive Care Med 1987;13:89–95
Hashimoto K, Miyamoto H, Suzuki K, Horikoshi S, Matsui M, Arai T, Kurosawa H. Evidence of organ damage after cardiopulmonary bypass. The role of elastase and vasoactive mediators. J Thorac Cardiovasc Surg 1992;104:666–673
Royston D, Fleming JS, Desai JB, Westaby S, Tayler KM. Increased production of peroxidation products associated with cardiac operations. Evidence for free radical generation. J Thorac Cardiovasc Surg 1986;91:759–766
Evans T, Carpenter A, Kinderman H, Cohen J. Evidence of increased nitric oxide production in patients with the sepsis syndrome. Circ Shock 1993;41:77–81
Suffredeni AF, Fromm RE, Parker MM, Brenner M, Kovacs JA, Wesley RA, Parrillo JE. The cardiovascular response of normal humans to the administration of endotoxin. N Engl J Med 1989;321:280–287
Nilsson L, Kulander L, Nystrom SO, Eriksson O. Endotoxins in cardiopulmonary bypass. J Thorac Cardiovasc Surg 1990;100:777–780
Radomski MW, Palmer RMJ, Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol 1987;92:181–187
Stamler JS, Simon DI, Jaraki O, Osborne JA, Francis S, Mullins M, Singel D, Loscalzo J. S-Nitrosylation of tissue-type plasminogen activator confers vasodilatory and antiplatelet propertics on the enzyme. Proc Natl Acad Sci USA 1992;89:8087–8091
Wright CD, Mulsch A, Busse R, Osswald H. Generation of nitric oxide by human neutrophil. Biochem Biophys Res Commun 1989;160:813–819
Niu X, Smith CW, Kubes P. Intercellular oxidative stress induced by nitric oxide synthesis inhibition increases endothelial cell adhesion to neutrophils. Circ Res 1994;74:1133–1140
Johnson D, Thomson D, Hurst T, Prasad K, Wilson T, Murphy F, Saxena A, Mayers I. Neutrophil-mediated acute lung injury after extracorporeal perfusion. J Thorac Cardiovasc Surg 1994;107: 1193–1202
Fukuyama N, Ichimori K, Su Z, Ishida H, Nakazawa H. Peroxynitrite formation from activated human leukocytes. Biochem Biophys Res Commun 1996;224:414–419
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hayashi, Y., Sawa, Y., Nishimura, M. et al. Role of nitric oxide in regulation of systemic vascular resistance during and after cardiopulmonary bypass. J Artif Organs 2, 152–156 (1999). https://doi.org/10.1007/BF02480059
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02480059