Abstract
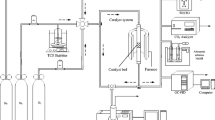
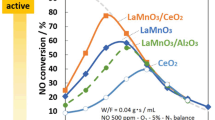
The total oxidation of CH3Cl, CH2Cl2 and ClH2C-CH2Cl has been investigated on a LaMnO3 perovskite type catalyst. Depending on the reaction temperature, a reversible deactivation of the catalyst was observed. Small amounts of by-products were formed at low reaction temperatures.
Similar content being viewed by others
References
J.J. Spivey, J.B. Butt:Catal. Today,11, 465 (1992).
T. Seiyama in: “Properties and Applications of Perovskite Type Oxides”, p. 215. Marcel Dekker Inc., New York 1992.
J.R. Kittrell, J.W. Eldridge, W.C. Conner:Catalysis,9, 162 (1991).
M. Wilde, K. Anders:Chem. Techn.,46, 316 (1994).
R. Schneider, D. Kiessling, M. Haftendorn, P. Kraak, M. Hackenberger, G. Wendt:Proc. 1st World Conf. on Environmental Catalysis, Pisa, Italy, 1995, p. 587.
C.F. Cullis D.E. Keene, D.L. Trimm:J. Catal.,19, 378 (1970).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schneider, R., Kiessling, D., Herzschuh, R. et al. Total oxidation of chlorinated hydrocarbons on LaMnO3 perovskite type catalyst. React Kinet Catal Lett 61, 245–250 (1997). https://doi.org/10.1007/BF02478379
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02478379