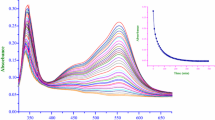
Abstract
The reaction under study is first order in the complex ion over the entire composition range of the mixed solvent. A minimum amount of mineral acid is needed for completion of the reaction (formation of colorless products), but the rate is practically acid-independent. The influence of solvent composition and some common ions on the reaction rate have been examined. A reaction mechanism consistent with the rate behavior is proposed where water is shown as an active participant in the dissociation process. A plausible explanation for the retardation effect of HSO4 − as against the accelerating effect of Cl− under water-scarce conditions is provided.
Similar content being viewed by others
References
F. Basolo, R.G. Pearson:Mechanisms of Inorganic Reactions, 2nd Ed., pp. 218-219. Wiley Eastern Limited, New Delhi.
P. Langer, S. Fallab, H. Erlenmeyer:Helv. Chim. Acta,37, 1050 (1954).
H. Brintriger, S. Fallab, H. Erlenmeyer:Helv. Chim. Acta,38, 557 (1955).
L. Seiden, F. Basolo, H.M. Neumann:J. Am. Chem. Soc.,81, 3809 (1959).
F.M. Van Meter, H.M. Neumann:J. Am. Chem. Soc.,98, 1388 (1976).
S. Tachiyashiki, H. Yametera:Polyhedron 2, 9 (1983).
R.D. Gillard, L.A.P. Kane-Maguire, P.A. Williams:J. Chem. Soc., Dalton Trans., 1792 (1977).
D.J. Farrington, J.G. Jones:J. Chem. Soc., Dalton Trans., 221 (1979).
K. Sriramam, J. Sreelakshmi, L. Ramadevi, Ch. Ramakrishna:Int. J. Chem. Kinet.,24, 919 (1992).
A.J. Riddick, W.B. Bunger:Organic Solvents, Physical Properties and Methods of Purification, 3rd Ed., p. 735. Wiley, New Delhi 1970.
A.I. Vogel:A Text Book of Quantitative Inorganic Analysis, 3rd Ed., p. 318. Longman 1975.
S. Kilpi, E. Lindell:Ann. Acad. Sci. Fennical. Ser., A.II, 136 (1967); Chem Abstr., 67 (1967)
T.S. Lee, I.M. Kolthoff, D.L. Leussing:J. Am. Chem. Soc.,79, 1286 (1957).
F. Basolo, J.C. Hayes, H.M. Neumann:J. Am. Chem. Soc.,76, 3807 (1954).
J.E. Dickens, F. Basolo, H.M. Neumann:J. Am. Chem. Soc.,79, 1286 (1957).
H.A. Laitinen, W.E. Harris:Chemical Analysis, McGraw Hill, 3rd Ed. p.23. Kogokusha, Tokyo 1975.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sriramam, K., Ramakrishna, C. & Sreelakshmi, J. Kinetics of dissociation of tris-1,10-phenanthroline-iron(II) complex cation in aqueous acetic acid solutions. React Kinet Catal Lett 61, 209–215 (1997). https://doi.org/10.1007/BF02477537
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02477537