Abstract
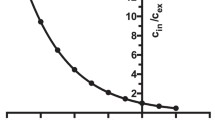
A membrane fraction, which contained dopamine receptors and heterotrimeric G proteins, was purified from homogenate of molluscan (Lymnaea) CNS tissues. Radioligand binding analysis with the use of [7.8-3H] dopamine detected the presence of a high-affinity binding site in this fraction. [7.8-3H] Dopamine was displaced in a dose-dependent manner by dopamine antagonists, S(-)-sulpiride, (±)-SKF83566, and fluphenazine. Radioligand binding analysis of purified membranes with the use of labelled GDP showed the presence of a high affinity binding site withB max=92±5 pmol/mg of protein andK d =64±10 nM. GDP, in contrast to GTP, markedly increased [7.8-3H] dopamine binding in the absence of metal cations (the maximum increase was 2.5-fold). Added separately, Na and Mg ions decreased the stimulatory influence of GDP. Jointly, these ions completely abolished this GDP influence on the [7.8-3H] dopamine binding. In the membrane fraction, GTPase activity in the presence of dopamine increased during an initial period and then decreased below the basal level. Therefore, we have demonstrated that in our experiments dopamine receptors in the purified membrane fraction are functionally coupled with heterotrimeric G proteins, but their interaction displays some specific features.
Similar content being viewed by others
References
I. Prudnikov, V. Tzyvkin, and T. Kastrykina, “GTP-dependent dopamine reception in the central nervous system tissue of the pond snailLymnaea stagnalis,”Neirofiziologiya,24, No. 6, 451–461 (1992).
I. Prudnikov, “Guanine nucleotide-induced enhancement of affinity of dopaminergic membrane receptors of nerve tissue ofLymnaea stagnalis for agonists,”Neirofiziologiya/Neurophysiology,25, No. 5, 271–278 (1993).
Y. Watanabe, T. Umegaki, and W. L. Smath, “Association of a solubilized prostaglandin E2 receptor from renal medulla with a pertussis toxin-reactive guanine nucleotide regulatory protein,”J. Biol. Chem.,261, 13430–13439 (1986).
M. Egelhaaf and P. R. Benjamin, “Coupled neuronal oscillators in the snailLymnaea stagnalis: endogenous cellular properties and network interactions,”J. Exp. Biol.,102, 93–114 (1983).
H. Pertoft and T. Laurent, “Isopycnic separation of cells and cell organelles by centrifugation in modified coloidal silica gradients,” in:Methods of Cell Separation, Vol. 1, N. Castimpoolas (ed.), Plenum Press, New York (1977), pp. 25–65.
E. Smart, Yun-shu Ying, and R. Anderson, “Hormonal regulation of caveolae internalization,”J. Mol. Cell Biol.,131, 929–938 (1995).
A. G. Splittgerbe and J. Sohl, “Nonlinearity in protein assays by the coomassie blue dye-binding method,”Anal. Biochem. 179, 198–201 (1989).
D. Cassel and Z. Selinger, “Catecholamine-stimulated GTPase activity in turkey erythrocyte membranes,”Biochim. Biophys. Acta,452, 538–551 (1976).
D. Brandt and E. Ross, “Catecholamine-stimulated GTPase cycle,”J. Biol. Chem.,261, 1656–1664 (1986).
B. Ceresa and L. Limbird, “Mutation of an aspartate residue highly conserved among G-protein-coupled receptors results in nonreciprocal disruption of α2 receptor-G-protein interactions,”J. Biol. Chem.,269, 29557–29564 (1994).
A. Gilman, “G proteins: transducer of receptor-generated signals,”Annu. Rev. Biochem.,56, 615–649 (1987).
D. Coleman, A. Berghuis, E. Lee, et al., “Structures of active conformations of G1αl and the mechanism of GTP hydrolysis,”Science,265, 1405–1412 (1994).
A. Fabiato and F. Fabiato, “Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells,”J. physiol.,75, 463–505 (1979).
A. E. Martell and R. M. Smith,Critical Stability Constants, Vol. 1, No. 2, Plenum, New York (1974).
T. Costa, J. Lang, C. Gless, and A. Herz, “Spontaneous association between opioid receptors and GTP-binding regulatory proteins in native membranes: specific regulation by antagonists and sodium ions,”Mol. Pharmacol.,37, 383–394 (1990).
G. Chin, E. Shapiro, S. Vogel, and J. Schwartz, “Aplysia synaptosomes. I. Preparation and biochemical and morphological characterizations of subcellular membrane fraction,”J. Neurosci.,9, 38–48 (1989).
J. M. Krauhs, L. A. Sordahl, and A. M. Brown, “Isolation of pigmented granules involved in extra-retinal photoreceptor inAplysia californica neurons,”Biochim. Biophys. Acta,471, No. 1, 25–31 (1977).
J. Stoof, T. de Vlieger, and J. Looder, “Opposing roles for D1 and D2 dopamine receptors in regulating the excitability of growth hormone producing cells in the snailLymnaea stagnalis,”Eur. J. Pharmacol.,106, 155–161 (1984).
T. Werkman, J. Lodder, T. de Vlieger, and J. Stoof, “Further pharmacological characterization of a D2-like dopamine receptor on growth hormone producing cells inLymnaea stagnalis,”Eur. J. Pharmacol.,139, 155–161 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Prudnikov, I.M., Tsyvkin, V.N. Membranes with dopamine receptors coupled to G proteins: Purification from theLymnaea central nervous system. Neurophysiology 30, 32–38 (1998). https://doi.org/10.1007/BF02463110
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02463110