Abstract
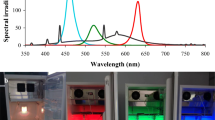
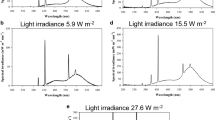
The germ tubes ofColletotrichum lagenarium showed negative phototropism to UV-blue (300–520 nm) and far-red (>700 nm) regions with maximum in the near ultraviolet (NUV) region, while monochromatic radiations of 575–700 nm (yellow-red region) induced positive phototropism with maximum in the red region. Green light (520–575 nm) was ineffective. Negative phototropism-inducing wavelength regions inhibited germ tube growth and positive phototropism-inducing wavelength regions promoted it significantly.Bipolaris oryzae did not show any phototropic response and light did not affect the germ tube growth. These results indicate that the lens effect, in combination with the light growth reaction and light growth inhibition, is the mechanism of the phototropism of germ tubes ofC. lagenarium. NUV radiation, which induced negative phototropism ofC. lagenarium, promoted appressorium formation, while red light, which induced positive phototropism, suppressed it significantly. In the case ofB. oryzae, light did not affect the infection structure formation. These results indicate that negative phototropism of germ tubes ofC. lagenarium favors the infection process by facilitating the contact of the tips of germ tubes with the host surface, while positive phototropism has the opposite effect.
Similar content being viewed by others
Literature cited
Banbury, G. H. 1959. Phototropism of lower plants. Encycl. Plant Physiol.17: 530–578.
Blaauw, A. H. 1914. Licht und Wachstum I. Z. Bot.6: 641–703.
Benedict, W. G. and Palmerley, R. A. 1979. Suppression of germ-tube abnormalities inHelminthosporium turcicum by blue light. Can. J. Bot.57: 87–89.
Buder, J. 1918. Die Inversion des Phototropismus beiPhycomyces. Ber. Dtsch. Bot. Ges.36: 104–105.
Calpouzos, L. and Lapis, D. B. 1970. Effect of light on pycnidium formation, sporulation, and tropism bySeptoria nodorum. Phytopathology60: 791–794.
Carlile, M. 1965. The photobiology of fungi. Annu. Rev. Plant Physiol.16: 175–202.
Castle, E. S. 1961. Phototropism, adaptation, and the light growth response ofPhycomyces. J. Gen. Physiol.45: 39–46.
Colhoun, J. 1973. Effects of environmental factors on plant disease. Annu. Rev. Phytopathol.11: 343–364.
Curry, G. M. and Gruen, H. E. 1957. Negative phototropism ofPhycomyces in the ultraviolet. Nature179: 1028–1029.
Curry, G. M. and Gruen, H. E. 1959. Action spectra for the positive and negative phototropism ofPhycomyces sporangiophores. Proc. Nat. Acad. Sci. USA45: 797–804.
Dey, P. K. 1933. Studies in the physiology of the appressorium ofColletotrichum gloeosporioides. Ann. Bot. (London)47: 305–312.
Emmett, R. W. and Parbery, G. D. 1975. Appressoria. Annu. Rev. Phytopathol.13: 147–167.
Forbes, I. L. 1939. Factors affecting the development ofPuccinia coronata in Louisiana. Phytopathology29: 659–684.
Fromme, F. D. 1915. Negative heliotropism of the uredinio-spore germ tubes ofPuccinia rhamni. Amer. J. Bot.2: 82–85.
Gäumann, E. 1946. Pflanzliche Infektionslehre. Verlag Birkhauser, Basel.
Gettkandt, C. 1954. Zur Kenntnis des Phototropismus der Keimmyzelien einiger parasitischer Pilze. Wiss. Z. Univ. Halle, Math. Nat. Kl.3: 691–710.
Homma, Y., Arimoto, Y. and Takahashi, H. 1983. Observation of the various growth stages of rice blast fungus (Pyricularia oryzae Cavara) by onion strip method. J. Pesticide Sci.8: 371–377.
Honda, Y. and Miyawaki, T. 1989. Phototropic response of conidium germ tubes inSeptoria obesa. Trans. Mycol. Soc. Japan30: 401–414.
Honda, Y., Kashima, T. and Kumagai, T. 1992. Suppression of brown spot disease of cultivated chrysanthemum by manipulating phototropic response of conidium germ tubes ofSeptoria obesa. J. Phytopathol.136: 270–278.
Koch, E. and Hoppe, H. H. 1987. Effect of light on uredospore germination and germ tube growth of soybean rust (Phakopsora pachyrhizi Syd.). J. Phytopathol.119: 64–74.
Koga, K., Sato, T. and Ootaki, T. 1984. Negative phototropism in the piloboloid mutants ofPhycomyces blakesleeanus Bgff. Planta162: 97–103.
Manachère, G. 1985. Sporophore differentiation of higher fungi: a survey of some actual problems. Physiol. Veg.23: 221–230.
Nicholson, R. L. 1984. Adhesion of fungi to the plant cuticle. In: Infection process of fungi, (ed. by Roberts, D. W. and Aist, J. R.), pp. 74–89. Rockefeller Foundation, New York.
Senger, H. and Schmidt, W. 1986. Cryptochrome and UV receptor: Diversity of photoreceptors. In: Photomorphogenesis in plants, (ed. by Kendrick, R. E. and Kronenberg, G. H.), pp. 137–158. Martinus Nijhoff, Dortrecht, The Netherlands.
Staples, R. C. and Macko, V. 1980. Formation of infection structures as a recognition response in fungi. Exp. Mycol.4: 2–16.
Tan, K. K. 1978. Light induced fungal development. In: The filamentous fungi (ed. by Smith, J. E. and Berry, D. R.), pp. 334–357. Edward Arnold, London.
Tsuru, T., Koga, K., Aoyama, H. and Ootaki, T. 1988. Optics inPhycomyces blakesleeanus sporangiophores relative to determination of phototropic orientation. Exp. Mycol.12: 302–312.
Welty, R. E. and Christensen, C. M. 1965. Negative phototropic growth ofAspergillus restrictus. Mycologia57: 311–313.
Xiao, J. Z., Watanabe, T., Kamakura, T., Ohshima, A. and Yamaguchi, I. 1994. Studies on cellular differentiation ofMagnaporthe grisea. Physicochemical aspects of substratum surfaces in relation to appressorium formation. Physiol. Mol. Plant Pathol.44: 227–236.
Yarwood, C. E. 1932. Reversal phototropism of the germ tubes of clover powdery mildew. Phytopathology22: 31. (Abstr.)
Author information
Authors and Affiliations
About this article
Cite this article
Islam, S.Z., Honda, Y. Influence of phototropic response of spore germ tubes on infection process inColletotrichum lagenarium andBipolaris oryzae . Mycoscience 37, 331–337 (1996). https://doi.org/10.1007/BF02461305
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02461305