Summary
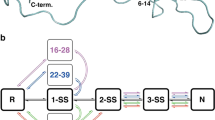
An amino-terminal extension of endothelin-l by the lys-Arg dipeptide in the prosequence (KR-ET-1) greatly increased the ratio of native-type to non-native-type disulfide isomer (96/4 versus 71/29) during the oxidative folding reaction. This improvement was completely abolished by substituting Asn for Asp at position 8 (D8N-KR-ET-1), whereas most of it was maintained with similar carboxamide analogues replaced at Glu10 or Asp18. Structure analyses by circular dichroism spectroscopy revealed that (i) in the carboxylate state, the α-helical content of the native-type isomer of KR-ET-l is higher than that of the native-type isomer of ET-1, while such a variation is not observed in the corresponding non-native-type isomer of KR-ET-l; and (ii) the enhanced α-helicity resulting from the Lys-Arg extension is largely diminished in D8N-KR-ET-l. From these results and our previous findings that the helical structure in KR-ET-l is stabilized by a particular salt bridge between the extended Arg−1 basic moiety and either the Asp8 or Glu10 acidic side chain in Et-1 [Aumelas, A. et al., Biochemistry, 34 (1995) 4546], we conclude that the formation of a specific salt bridge between the side chains of Arg−1 and Asp8 in KR-ET-1 is critical for the predominant generation of the native-type disulfide isomer, probably because it stabilizes the helical structure of parental ET-1.
Similar content being viewed by others
References
Yanagisawa, M., Kurihara, H., Kimura, S., Tomobe, Y., Kobayashi, M., Mitsui, Y., Yazaki, Y., Goto, K. and Masaki, T., Nature, 332 (1988) 411.
Kumagaye, S., Kuroda, H., Nakajima, K., Watanabe, T.X., Kimura, T., Masaki, T. and Sakakibara, S., Int. J. Pept. Protein Res., 32 (1988) 519.
Itoh, Y., Yanagisawa, M., Ohkubo, S., Kimura, C., Kosaka, T., Inoue, A., Ishida, N., Mitsui, Y., Onda, H., Fujino, M. and Masaki, T., FEBS Lett. 231 (1988) 440.
Kitada, C., Tanaka, E. and Fujino, M., In Shimonishi, Y. (Ed.) Peptide Chemistry 1990, Protein Research Foundation, Osaka, Japan, 1991, pp. 405–408.
Galantino, M., de Castiglione, R., Cristiani, C., Vaghi, F., Liu, W., Zhang, J.-W and Tam, J.P., Pept. Res. 8 (1995) 154.
Nomizu, M., Inagaki, Y., Iwamatsu, A., Kashiwabara, T., Ohta, H., Morita, A., Nishikori, K., Otaka, A., Fujii, N. and Roller, P.P., Int. J. Pept. Protein Res., 38 (1991) 580.
Nakajima, K., Kubo, S., Kumagaye, S., Nishio, H., Tsunemi, M., Inui, T., Kuroda, H., Chino, N., Watanabe, T.X., Kimura, T. and Sakakibara, S., Biochem Biophys. Res. Commun., 163 (1989) 424.
Aumelas, A., Chiche, L., Kubo, S., Chino, N., Tamaoki, H. and Kobayashi, Y., Biochemistry, 34 (1995) 4546.
Tamaoki, H., Kobayashi, Y., Nishimura, S., Ohkubo, T., Kyogoku, Y., Nakajima, K., Kumagaye, S., Kimura, T. and Sakakibara, S., Protein Eng., 4 (1991) 509.
Aumelas, A., Chiche, L., Mahe, E., Le-Nguyen, D., Sizum, P., Berthault, P. and Perly, B., Int. J. Pept. Protein Res., 37 (1991) 315.
Anderson, N.H., Chen, C., Marchner, T.M., Krystek Jr., S.R. and Bassolino, D.A., Biochemistry. 31 (1992) 1280.
Creighton, T.E., J. Mol. Biol., 144 (1980) 521.
Moroder, L., Besse, D., Musiol, H.-J., Rudolph-Böhner, S. and Siedler, F., Biopolymers, 40 (1996) 207.
Kubo, S., Chino, N., Watanabe, T.X., Kimura, T. and Sakakibara, S., Pept. Res., 6 (1993) 66.
Kubo, S., Chino, N., Kimura, T. and Sakakibara, S., Biopolymers 38 (1996) 733.
Ikemura, H., Takagi, H. and Inouye, M., J. Biol. Chem., 262 (1987) 7859.
Shinde, U., Li, Y., Chatterjee, S. and Inouye, M., Proc. Natl. Acad. Sci. USA, 90 (1993) 6924.
Silen, J.L. and Agard, D.A., Nature, 341 (1989) 362.
Winther, J.R. and Sorensen, P., Proc. Natl. Acad. Sci. USA, 88 (1991) 9330.
Weissman, J.S. and Kim, P.S., Cell, 71 (1992) 841.
Ohashi, H., Katsuta-Enomoto, Y., Yasufuku, K., Okada, K. and Yano, M., J. Biochem., 110 (1991) 628.
Tam, J.P., Dong, X. and Wu, C.-R., In Yanaihara, N. (Ed.) Peptide Chemistry 1992 (Proceedings of the 2nd Japan Symposium on Peptide Chemistry), ESCOM, Leiden, The Netherlands, 1993, pp. 24–26.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kubo, S., Chino, N., Nakajima, K. et al. Improvement in the oxidative folding of endothelin-1 by a lys-Arg extension at the amino terminus: Implication of a salt bridge between Arg−1 and Asp8 . Lett Pept Sci 4, 185–192 (1997). https://doi.org/10.1007/BF02443532
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02443532