Summary
Previous studies have shown that differences in nuclear morphology are generally sufficient to determine the species origin of cells in interspecific grafts between the Japanese quail and domestic chicken. Most quail nuclei possess 1–3 large nucleolus-associated masses of heterochromatin. Chick cells, on the other hand, usually present a more diffuse, stippled distribution of nuclear heterochromatin.
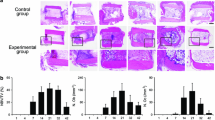
Quail embryonic limb rudiments, some with and some without established marrow cavities, were explanted and grown on the chorioallantoic membrane of the chick. Three to five days post-grafting, the explants were surgically fractured and allowed to heal. Tissues were collected and histologically processed during the latter period.
The fractures healed completely within 5–6 days and no callus was established in the process. The nuclear staining pattern of the osteoblasts and osteocytes throughout the rudiments and at the fracture site indicated that they were derived from the graft. Possible sources for these cells included the periosteum, endosteum, and posthypertrophy chondrocytes. By contrast, most of the nuclei in the osteoclasts were chick-like and were apparently derived from cells originating in the host. Because the quail-like heterochromatin marker was normally present in a small number (2.5%) of chick osteoclast nuclei and was lacking in about 5% of native quail osteoclast nuclei, the precise extent of the participation of donor, i.e., quail bone and marrow stromal cells in osteoclast formation, could not be determined. However, the data suggest that in large measure the precursor cells for most osteoclasts were hematogenously derived and were carried to the grafted rudiments by the blood vascular system.
Similar content being viewed by others
References
Tonna, E.A., Cronkite, E.P.: Cellular response to fracture studied with tritiated thymidine, J. Bone Joint Surg.43A:352–362, 1961
Hall, B.K., Jacobson, H.N.: The repair of fractured membrane bones in newly hatched chicks, Anat. Rec.181:55–70, 1975
Kernek, C.B., Wray, J.B.: Cellular proliferation in the formation of fracture callus in the rat tibia, Clin. Orthop.91:197–209, 1973
Ketenjian, A.U., Arsenis, C.: Morphological and biochemical studies during differentiation and calcification of fracture callus cartilage, Clin. Orthop.197:266–273, 1975
Kuhlman, R.E., McNamee, M.J.: The biochemical importance of the hypertrophic cartilage cell area to endochondral bone formation, J. Bone Joint Surg.52A:1025–1032, 1970
Göthlin, G., Ericsson, J.L.E.: Observations on the mode of uptake of thorium dioxide particles by osteoclasts in fracture callus, Calcif. Tissue Res.10:216–227, 1972
Fischman, D.A., Hay, E.D.: Origin of osteoclasts from mononuclear leucocytes in regenerating newt limbs. Anat. Rec.143:329–334, 1962
Buring, K.: The origin of cells in heterotopic bone formation, Clin. Orthop.110:293–302, 1975
Kahn, A.J., Simmons, D.J.: Investigation of cell lineage in bone using a chimera of chick and quail embryonic tissue, Nature258:325–327, 1975
Le Douarin, N.: A biological cell labeling technique and its use in experimental embryology, Dev. Biol.30:217–222, 1973
Jotereau, F.V., Le Douarin, N.M.: The developmental relationship between osteocytes and osteoclasts. A study using the quail-chick nuclear marker in endochondral ossification, Dev. Biol.63:253–265, 1978
Patt, H.M., Maloney, M.A.: Bone marrow regeneration after local injury: a review, Exp. Hematol.3:135–146, 1975
Holtrop, M.: The origin of bone cells in endochondral ossification. In H. Fleisch (ed.): Calcified Tissues, pp. 32–35, Springer-Verlag, New York, 1966
Shimomura, U., Wezeman, F.H., Ray, R.D.: The growth cartilage plate of the rat rib: cellular differentiation, Clin. Orthop.90:246–254, 1973
Crelin, E.S., Koch, W.E.: An autoradiographic study of chondrocyte transformation into chondroclasts and osteocytes during bone formationin vitro, Anat. Rec.158:473–484, 1967
Kahn, A.J., Simmons, D.J.: Chondrocyte-to-osteocyte transformation in grafts of perichondrium-free epiphyseal cartilage, Clin. Orthop.129:299–304, 1977
Hamburger, V. Hamilton, H.L.: A series of normal stages in the development of the chick embryo, J. Morphol.88:49–67, 1951
Bucher, O.: Untersuchunger uber die Regenerationsvorgange an Experimentell Gesetzten Knochenbruchen in der Kultur in Vitro, Acta Anat. (Basel)14:98–107, 1952
Krull, G.: Untersuchungen uber Frakturheilung in der Gewebekultur, Arch. Orthop. Unfallchir.37:131–137, 1936
Prasad, G.C., Reynolds, J.J.: Effect of environmental factors on the repair of bonein vitro, J. Bone Joint Surg.50B:401–408, 1968
Niven, J.S.F.: The repairin vitro of embryonic skeletal rudiments after experimental injury, J. Pathol. Bacteriol.134:307–324, 1931
Sevastikoglou, J.A.: Morphological studies of fracture healing in tissue culture, Acta Orthop. Scand.32:199–209, 1962
Amsell, S., Dell, E.S.: Bone formation by hemopoietic tissue. Separation of preosteoblasts from hemopoietic stem cell function in the rat, Blood39:267–273, 1972
Knospe, W.H., Gregory, S.A., Husseni, S.G., Fried, W., Trobaugh, F.E., Jr.: Origin and recovery of colony-forming units in locally curetted bone marrow of mice, Blood30:331–340, 1972
Friedenstein, A.J.: Determined and inducible osteogenic precursor cells. Hard tissue growth and repair, Ciba Found. Symp.11:169–185, 1973
Friedenstein, A.J., Chailakhjan, R.K., Lalykina, K.S.: The development of fibroblast colonies in monolayer cultures of guinea pig bone marrow and spleen cells, Cell Tissue Kinet.3:393–403, 1970
Friedenstein, A.J., Piatetzky-Shapiro, I.I., Petrakova, K.V.: Osteogenesis in transplants of bone marrow cells, J. Embryol. Exp. Morphol.16:381–390, 1966
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Simmons, D.J., Kahn, A.J. Cell lineage in fracture healing in chimeric bone grafts. Calcif Tissue Int 27, 247–253 (1979). https://doi.org/10.1007/BF02441193
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02441193