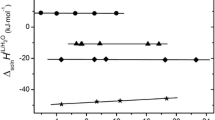
Abstract
The solubilities of potassium fluoride, chloride, and bromide in ethanol, formamide, and N-methylformamide and in binary mixtures of these solvents were determined at 25°C. The standard molar Gibbs energies of solution, Δsoln G o, in the neat solvents were related to their hydrogen bonding abilities. The values of Δsoln G o in the mixtures were fitted with expressions of the quasilattice quasichemical theory, and the preferential solvation of the ions was thereby established.
Similar content being viewed by others
References
A.-K. S. Labban and Y. Marcus,J. Solution Chem. 20, 221 (1991).
Y. Marcus,Introduction to Liquid State Chemistry (Wiley, Chichester, 1977), p. 243.
C. W. Davies,J. Chem. Soc. 2093 (1938).
C. W. Davies,Ion Association (Butterworths, London, 1962), p. 39.
Y. Marcus,J. Chem. Soc., Faraday Trans. 1 84, 1465 (1988).
T. A. Zaichikova, V. V. Toropov, E. V. Parfenyuk, and G. A. Krestov,Zhur. Fiz. Khim. 61, 2564 (1987).
R. Reynaud,Compt. Rend. 270C, 1813 (1970).
C. de Visser and G. Somsen,J. Solution Chem. 3, 847 (1974); C. de Visser, W. J. M. Heuvelsland and G. Somsen,J. Solution Chem. 4, 311 (1975).
J. A. Riddick, W. B. Bunger, and T. K. Sakano,Organic Solvents, 4th edn. (Wiley-Interscience, New York), 1986.
Y. Marcus, G. Hefter, and T.-S. Pang,J. Chem. Soc. Faraday Trans. 90, 1899 (1994).
C. M. Kinart and W. J. Kinart,Polish J. Chem. 68, 349 (1994).
K. Quitzsh, H.-P. Hofmann, R. Pfestorf, and G. Geiseler,Z. Phys. Chem. 235, 99 (1960).
L. Pikkarainen,Thermochim. Acta 178, 311 (1991).
M. Bloemendal and G. Somsen,J. Solution Chem. 17, 1067 (1988).
C. A. L. DeBruyn,Rec. Trav. Chim. 11, 29, 112 (1892);Z. Phys. Chem. 10, 781 (1892); quoted in Ref. 27.
W. E. S. Turner and C. C. Bissett,J. Chem. Soc. 103, 1904 (1913).
R. P. Stewart and W. C. Schumb,J. Am. Chem. Soc. 52, 3962 (1930).
E. R. Kirn and H. L. Dunlap,J. Am. Chem. Soc. 53, 391 (1931).
F. G. Germuth,J. Franklin Inst. 212, 346 (1931); quoted in Ref. 27.
G. W. Ferner and M. G. Mellon,Ind. Eng. Chem. Anal. Ed. 6, 345 (1934).
R. G. Larson and H. Hunt,J. Phys. Chem. 43, 417 (1939).
A. J. Parker and R. Alexander,J. Am. Chem. Soc. 90, 3313 (1968); R. Alexander, A. J. Parker, J. H. Sharp, and W. E. Waghorne, ibid.J. Am. Chem. Soc.,94, 1148 (1972).
B. Scrosati and C. A. Vincent, eds.Solubility Data Series, Vol. 11 (Pergamon, Oxford, 1980); recommended values.
G. T. Hefter,Rev. Inorg. Chem. 10, 185 (1989); quoting Ref. 26.
O. Chiavone-Filho and P. Rasmussen,J. Chem. Eng. Data 38, 367 (1993).
W. F. Linke,Solubilities of Inorganic and Metal-Organic Compounds, 4th edn. (Am. Chem. Soc., Washington, 1965).
H. Stephen and T. Stephen,Solubilities of Inorganic and Organic Compounds (Pergamon, Oxford, 1963).
I. P. Nikitina, B. S. Krumgalz, D. G. Traber, and G. F. Fedotova,Zhur. Neorg. Khim. 14, 2593 (1969).
D. D. Wagman, W. H. Evans, V. B. Parker, R. H. Schumm, I. Halow, S. M. Bailey, K. L. and R. L. Nutall,J. Phys. Chem. Ref. Data 11, Suppl. 2, 1982 (The NBS tables of chemical thermodynamic properties).
Y. Marcus, M. J. Kamlet, and R. W. Taft,J. Phys. Chem. 92, 3613 (1988).
G. T. Hefter and P. J. McLay,J. Solution Chem. 17, 535 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Labban, AK.S., Marcus, Y. The solubility and solvation of salts in mixed nonaqueous solvents. 2. Potassium halides in mixed protic solvents. J Solution Chem 26, 1–12 (1997). https://doi.org/10.1007/BF02439440
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02439440