Summary
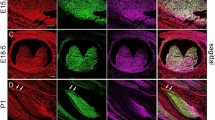
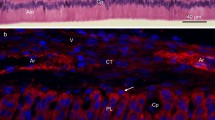
Several extracellular matrix components (procollagen type III, fibronectin, collagen type IV, laminin and nidogen) and microfilament constituents (actin, α-actinin and vinculin) were localized by indirect immunofluorescence microscopy in frozen sections of embryonic mouse molars. Nidogen was present at the epithelio-mesenchymal junction during polarization and initial steps of functional differentiation of odontoblasts. Nidogen disappeared at a stage where direct contacts between preameloblasts and predentin were required to allow the initiation of ameloblast polarization. Our observations concerning the distribution of procollagen type III and fibronectin during odontoblast differentiation add to current knowledge. Procollagen type III and fibronectin surrounding preodontoblasts accumulated at the apical part of polarizing and functional odontoblasts secreting “initial” predentin. Procollagen type III, but not fibronectin, disappeared in front of functional odontoblasts synthesizing “late” predentin and dentin. Fibronectin, present in “initial” predentin, was no longer detected in “late” predentin and dentin but was found between odontoblasts secreting “late” predentin and dentin. Actin, α-actinin and vinculin were concentrated in the peripheral cytoplasm of preameloblasts and accumulated at the apical and basal poles of functional ameloblasts. During differentiation of odontoblasts, the three proteins accumulated at the apical pole of these cells. Time and space correlations between matrix and microfilament modifications during odontoblast and ameloblast differentiation are documented. The possibility is discussed that there is transmembranous control of the cytoskeletal activities of odontoblasts and ameloblasts by the extracellular matrix.
Similar content being viewed by others
References
Ben Ze'ev A (1986) The relationship between cytoplasmic organization gene expression and morphogenesis. Trends Biochem Sci 11:478–481
Burridge K (1986) Substrate adhesions in normal and transformed fibroblasts: organization and regulation of cytoskeletal, membrane and extracellular matrix components at focal contacts. Cancer Rev 4:18–78
Burridge K, Connell L (1983) Talin: a cytoskeletal component concentrated in adhesion plaques and other sites of actin-membrane interaction. Cell Motil 3:405–417
Burridge K, Feramisco JR (1980) Microinjection and localization of a 130 K protein in living fibroblasts: a relationship to actin and fibronectin. Cell 19:587–595
Butler WT, Sato S, Rahemtulla F, Prince CW, Tomana M, Bhown M, Dinuzio MT, Bronckers ALJJ (1984) Glycoproteins of bone and dentin. In: Butler WT (ed) The chemistry and biology of mineralized tissues. Ebsco Media, Birmingham, Alabama, pp 107–112
Chen WT, Hasegawa E, Hasegawa T, Weinstock C, Yamada KM (1985) Development of cell surface linkage complexes in cultured fibroblasts. J Cell Biol 100:1103–1114
Drenckhahn D, Franz H (1986) Identification of actin, α-actinin and vinculin-containing plaques at the lateral membrane of epithelial cells. J Cell Biol 102:1843–1852
Geiger B (1983) Membrane-cytoskeleton interaction. Biochim Biophys Acta 737:305–341
Geiger B, Avnur Z, Rinnerthaler G, Hinssen H, Small JV (1984) Microfilament-organizing centers in areas of cell contact: cytoskeletal interactions during cell attachment and locomotion. J Cell Biol 99:83s-91s
Geiger B, Volk T, Volberg T (1985) Molecular heterogeneity of adherens junctions. J Cell Biol 101:1523–1531
Hill CS, Lemanski LF (1985) Immunoelectron microscopic localization of α-actinin and actin in embryonic hamster heart cells. Eur J Cell Biol 39:300–312
Horwitz A, Duggan K, Buck C, Beckerle MC, Burridge K (1986) Interaction of plasma membrane fibronectin receptor with talin: a transmembrane linkage. Nature 320:531–533
Hughes RC, Butters TD, Aplin JD (1981) Cell surface molecules involved in fibronectin-mediated adhesion. A study using specific antisera. Eur J Cell Biol 26:198–207
Kallenbach E, Piesco NP (1978) The changing morphology of the epithelium-mesenchyme interface in the differentiation zone of growing teeth of selected vertebrate and its relationship to possible mechanisms of differentiation. J Biol Buccale 6:229–240
Karcher-Djuricic V, Staubli A, Meyer JM, Ruch JV (1985) Acellular dental matrices promote functional differentiation of ameloblasts. Differentiation 29:169–175
Katchburian E, Burgess AMC (1983) Lysosomes and removal of the basal lamina of ameloblasts in early stages of odontogenesis. Cell Biol Int Rep 7:407–415
Kemp RB, Hinchliffe J (eds) (1984) Matrices and cell differentiation Liss, New York
Koteliansky VE, Gneushev GN, Belkin AM (1985) Purification of a 175-kDa membrane protein, its localization in smooth and cardiac muscles. Interaction with the cytoskeletal protein-vinculin. FEBS Lett 182:67–72
Lau EC, Boukari A, Arechaga J, Osman M, Ruch JV (1983)35S-autoradiography study of sulfated GAG accumulation and turnover in embryonic mouse tooth germs. J Craniofac Genet Dev Biol 3:117–131
Lazarides E, Burridge K (1975) α-actinin: immunofluorescent localization of a muscle structural protein in nonmuscle cells. Cell 6:289–298
Lehto UP, Virtanen I (1985) Vinculin in cultured bovine lensforming cells. Cell Differ 16:153–160
Lesot H (1981) Collagen type I trimer synthesis by cultured embryonic mouse molars. Eur J Biochem 116:541–546
Lesot H (1986) Cell-matrix interactions during odontoblast differentiation. Front Matrix Biol 11:139–159
Lesot H, Karcher-Djuricic V, Ruch JV (1981a) Synthesis of collagen type I, type I trimer and type III by embryonic mouse dental epithelial and mesenchymal cells in vitro. Biochim Biophys Acta 656:206–212
Lesot H, Osman M, Ruch JV (1981b) Immunofluorescent localization of collagens, fibronectin and laminin during terminal differentiation of odontoblasts. Dev Biol 82:371–381
Lesot H, Meyer JM, Ruch JV, Weber K, Osborn M (1982) Immunofluorescent localization of vimentin, prekeratin and actin during odontoblast and ameloblast differentiation. Differentiation 21:133–137
Lesot H, Karcher-Djuricic V, Mark M, Meyer JM, Ruch JV (1985) Dental cell interaction with extracellular-matrix consituents: type I collagen and fibronectin. Differentiation 29:176–181
Lesot H, Smith AJ, Meyer JM, Staubli A, Ruch JV (1986) Cell-matrix interactions: influence of noncollagenous proteins from dentin on cultured dental cells. J Embryol Exp Morphol 96:195–209
Linde A (1984) Dynamic aspects of dentinogenesis. In: Butler WT (ed) The chemistry and biology of mineralized tissues. Ebsco Media, Birmingham, Alabama, pp 344–355
Linde A, Johansson S, Jonsson R, Jontell M (1982) Localization of fibronectin during dentinogenesis in rat incisor. Arch Oral Biol 27:1069–1073
Mangeat P, Burridge K (1984) Actin-membrane interaction in fibroblasts: what proteins are involved in this association?. J Cell Biol 99:95s-103s
Meyer JM, Staubli A, Ruch JV (1981) Ruthenium red staining and tannic acid fixation of dental basement membrane. Cell Tissue Res 220:589–597
Nishikawa S, Kitamura H (1986) Localization of actin during differentiation of the ameloblast, its related epithelial cells and odontoblasts in the rat incisor using NBD-phallacidin. Differentiation 30:237–243
Nowack H, Gay S, Wick G, Becker U, Timpl R (1976) Preparation and use in immunohistology of antibodies specific for type I and type III collagen and procollagen. J Immunol Methods 12:117–124
Osman M, Ruch JV (1981)3H-glucosamine and3H-proline radio-autography of embryonic mouse dental basement membrane. J Craniofac Genet Dev Biol 1:95–108
Pitaru S, Aubin JE, Bhargava U, Melcher AH (1987) Immunoelectron microscopic studies on the distributions of fibronectin and actin in a cellular dense connective tissue: the periodontal ligament of the rat. J Periodont Res 22:64–74
Ruch JV (1984) Tooth morphogenesis and differentiation. In: Linde A (ed) Dentin and dentinogenesis. CRC Press, Boca Raton, pp 47–49
Ruch JV (1985) Odontoblast differentiation and the formation of the odontoblast layer. J Dent Res 64:489–498
Ruch JV, Fabre M, Karcher-Djuricic V, Staubli A (1974) The effects of L-azetidine-2-carboxylic acid (analogue of proline) on dental cytodifferentiation in vitro. Differentiation 2:211–220
Ruch JV, Karcher-Djuricic V, Staubli A, Fabre M (1975) Effet de la cytochalasine et de la colchicine sur les cytodifférenciations dentaires in vitro. Arch Anat Microsc Morphol Exp 64:113–134
Ruoslahti E, Engvall E, Hayman EG (1981) Fibronectin: current concepts of its structure and functions. Coll Relat Res 1:95
Singer II, Paradiso PR (1981) A transmembrane relationship between fibronectin and vinculin (130 kD protein): serum modulation in normal and transformed hamster fibroblasts. Cell 24:481–492
Singer II, Kazazis DM, Kawka DW (1985) Localization of the fibronexus at the surface of granulation tissue myofibroblasts using double-label immunogold electron microscopy on ultrathin frozen sections. Eur J Cell Biol 38:94–101
Slavkin HC (1974) Embryonic tooth formation A tool for developmental biology. In: Melcher AH (ed) Oral sciences reviews. Munksgaard, Copenhagen
Slavkin HC, Bringas P (1976) Epithelial-mesenchyme interactions during odontogenesis. IV. Morphological evidence for direct heterotypic cell-cell contacts. Dev Biol 50:428–442
Thesleff I, Hurmerinta K (1981) Tissue interactions in tooth development. Differentiation 18:75–88
Thesleff I, Barrach HJ, Foidart JM, Vaheri A, Pratt RM, Martin GR (1981) Changes in the distribution of type IV collagen, laminin, proteoglycan and fibronectin during mouse tooth germ development. Dev Biol 81:182–192
Timpl R, Martin GR, Bruckner P, Wick G, Wiedemann H (1978) Nature of the collagenous protein in a tumor basement membrane. Eur J Biochem 84:43–52
Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR (1979a) Laminin-A glycoprotein from basement membranes. J Biol Chem 254:9933–9937
Timpl R, Glanville RW, Wick G, Martin GR (1979b) Immunochemical study on basement membrane (type IV) collagens. Immunology 38:109–116
Timpl R, Dziadek M, Fujiwara S, Nowack H, Wick G (1983) Nidogen: a new, self-aggregating basement membrane protein. Eur J Biochem 137:455–465
Turksen K, Kalnins VI (1987) The cytoskeleton of chick retinal pigment epithelial cells in situ. Cell Tissue Res 248:95–101
Ungar F, Geiger B, Ben-Ze'ev A (1986) Cell contact-and shape-dependent regulation of vinculin synthesis in cultured fibroblasts. Nature 319:787–791
Watt FM (1986) The extracellular matrix and cell shape. Trends Biochem Sci 11:482–485
Wilkins JA, Chen KY, Lin S (1983) Detection of high molecular weight vinculin binding proteins in muscle and non muscle tissues with an electroblot-overlay technique. Biochem Biophys Res Commun 116:1026–1032
Yamada KM, Kennedy DW, Kimata K, Pratt RM (1980) Characterization of fibronectin interactions with glycosaminoglycans and identification of active proteolytic fragments. J Biol Chem 255:6055
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kubler, MD., Lesot, H. & Ruch, J.V. Temporo-spatial distribution of matrix and microfilament components during odontoblast and ameloblast differentiation. Roux’s Arch Dev Biol 197, 212–220 (1988). https://doi.org/10.1007/BF02439428
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02439428