Abstract
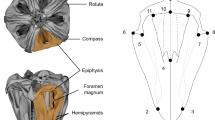
Fluctuating asymmetry (FA) is generally viewed as a population-level character. It is described by some measure of the variance of the difference between the right and left sides for a collection of individuals. Very little is known of the developmental origins of FA, despite the fact that FA is widely used to estimatedevelopmental stability. We present a novel technique for examining the growth trajectory of the asymmetries that give rise to FA, and we explore two sample data sets for the brachyuran crabHemigrapsus nudus. We have traced the fate of these small, random deviations from perfect symmetry through three successive molts of individual crabs. Invertebrates that molt, and hence grow in discrete steps, provide an easily preserved record of their growth. Model II regressions of measurements from one molt versus the previous molt can help describe the stability of subtle departures from symmetry over time. Although any number of different patterns may occur, we identify four general cases: a) asymmetries vary at random throughout growth (random determination), b) asymmetries remain unchanged in sign and magnitude (constant asymmetries), c) asymmetries increase in proportion to character size and hence increase with growth (size-dependent asymmetries), and d) asymmetries persist, but are reduced in magnitude (damped asymmetries). Data from tenHemigrapsus nudus, measured for between 21 and 28 metrical, limb-segment characters over three successive molts, yielded associations most similar to our pattern ‘b’, although some subtle departures in the direction of pattern ‘c’ were also observed. Persistent asymmetries accounted for 26% and 20% of the variance among asymmetries between molts 1 and 2, and molts 2 and 3 respectively. Thus, in spite of large and rapid increments in the external size of the crab, these subtle asymmetries tended to persist in both direction and magnitude, from molt to molt. This result suggests either i) that individual crabs have a genetic predisposition towards asymmetry in a particular direction but contribute to a continuous and normal distribution ofR-L differences at the population level, or ii) that these subtle asymmetries arose at some earlier ontogenetic stage and were preserved through growth. Either interpretation has important ramifications for the study of FA. The first suggests that under some circumstances FA may not provide a valid measure of developmental instability, because subtle departures from symmetry in an individual may have a genetic basis. The second implies that subtle departures from bilateral symmetry are not ‘corrected’ as an individual grows.
Similar content being viewed by others
References
Angus, R. A. & R. H. Schultz, 1983. Meristic variation in homozygous and heterozygous fish. Copeia 1983: 287–299.
Coyne, J. A., 1987. Lack of response to selection for directional asymmetry inDrosophila melanogaster. J. Hered. 78: 119.
Hartnoll, R. G., 1982. Growth, pp. 111–196 in The Biology of Crustacea, edited by L. G. Abele. Academic Pr., London.
LaBarbera, M. L., 1989. Analyzing body size as a factor in ecology and evolution. Ann. Rev. Ecol. Syst. 20: 97–117.
Leamy, L., 1984. Morphometric studies in inbred and hybrid house mice. V. Directional and fluctuating asymmetry. Amer. Nat. 123: 579–593.
Leary, R. F. & F. W. Allendorf, 1989. Fluctuating asymmetry as an indicator of stress in conservation biology. Trends Ecol. Evol. 4: 214–217.
Livshits, G., L. Davidi, E. Kobyliansky, D. Ben-Amital, Y. Levi & P. Merlob, 1988. Decreased developmental stability as assessed by fluctuating asymmetry of morphometric traits in preterm infants. Amer. J. Med. Gen. 29: 793–805.
Ludwig, W., 1932. Das Rechts-Links Problem im Teirreich und beim Menschen. Springer, Berlin. 496.
Mather, K., 1953. Genetical control of stability in development. Heredity 7: 297–336.
Maynard Smith, J. & K. C. Sondhi, 1960. The genetics of a pattern. Genetics 45: 1039–1050.
McKenzie, J. A. & G. M. Clarke, 1988. Diazinon resistance, fluctuating asymmetry and fitness in the Australian sheep blowfly,Lucilia cuprina. Genetics 120: 213–220.
McVean, A., 1982. Autotomy, pp. 107–132 in The Biology of Crustacea, edited by D. E. Bliss. Academic Pr., New York.
Møller, A. P. & J. Höglund, 1992. Patterns of fluctuating asymmetry in avian feather ornaments: Implications for models of sexual selection. Proc. Roy. Soc. Lond. B245: 1–5.
Neville, A. C., 1976. Animal Asymmetry. Arnold (Inst. Biol. Stud. Biol.), London. 60.
Palmer, A. R. & C. Strobeck, 1986. Fluctuating asymmetry: measurement, analysis, patterns. Ann. Rev. Ecol. Syst. 17: 391–421.
Palmer, A. R. & C. Strobeck, 1992. Fluctuating asymmetry as a measure of developmental stability: Implications of non-normal distributions and power of statistical tests. Acta Zool. Fenn. 191: 57–72.
Palmer, A. R., C. Strobeck & A. K. Chippindale, 1993. Bilateral variation and the evolutionary origin of macroscopic asymmetries. Genetica (this volume).
Parsons, P. A., 1990. Fluctuating asymmetry: An epigenetic measure of stress. Biol. Rev. 65: 131–145.
Price, T., E. Chi, M. Pavelka & M. Hack, 1991. Population and developmental variation in the feather tip. Evolution 45: 518–533.
Rice, W. R., 1989. Analyzing tables of statistical tests. Evolution 43: 223–225.
Sokal, R. R. & J. F. Rohlf, 1981. Biometry. Freeman, San Francisco, CA. 859.
Strawn, K., 1961. A comparison of meristic means and variances of wild and laboratory-raised samples of the fishes,Etheostoma grahami andE. lepidum (Percidae). Texas J. Sci. 13: 127–159.
Taning, A., 1952. Experimental study of meristic characters in fishes. Biol. Rev. 27: 169–193.
Tuinstra, E. J., G. Dejong & W. Scharloo, 1990. Lack of response to family selection for directional asymmetry inDrosophila melanogaster: Left and right are not distinguished in development. Proc. Roy. Soc. Lond. B 241: 146–152.
VanValen, L., 1962. A study of fluctuating asymmetry. Evolution 16: 125–142.
Wooten, M. C. & M. H. Smith, 1986. Fluctuating asymmetry and genetic variability in a natural population ofMus musculus. J. Mammal. 67: 725–732.
Zakharov, V. M., 1992. Population phenogenetics: Analysis of developmental stability in natural populations. Acta Zool. Fenn. 191: 7–30.
Zhivotovsky, L. A., 1992. A measure of fluctuating asymmetry for a set of characters. Acta Zool. Fenn. 191: 37–77.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chippindale, A.K., Palmer, A.R. Persistence of subtle departures from symmetry over multiple molts in individual brachyuran crabs: Relevance to developmental stability. Genetica 89, 185–199 (1993). https://doi.org/10.1007/BF02424513
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02424513