Summary
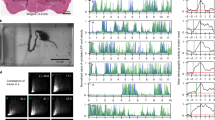
Single neurons were simultaneously recorded in the forepaw areas of the primary somatosensory (SI) cortex and ventroposterolateral (VPL) thalamus of awake rats during rest and running behaviors. Movement dependent changes in somatic sensory transmission were tested by generating post-stimulus histograms of these neurons' responses to stimulation through electrodes chronically implanted under the skin of the forepaw, while the aminal ran on a timed treadmill. As viewed in post-paw-stimulus histograms, the evoked unit responses (EURs) could be differentiated into short (4.5 ± 0.1−10.9 ± 0.2 ms) and longer (12.9 ± 0.4 31.3 ± ± 0.9 ms) latency components (“SEURs” and “LEURs”, respectively). The magnitudes of firing during these responses were measured and normalized as percent increases over background firing. By comparison with resting behavior, treadmill movement suppressed both SEURs and LEURs in the thalamus, as well as the cortex. The SEURs, however, were much more strongly suppressed in the SI cortex (−48.3 ± 2.7%) than in the VPL thalamus (−28.1 ± 6.7%). By contrast, similar magnitudes of suppression of LEURs were found in the SI (−25.8 ± 8.6%) and VPL (−26.5 ± 11.1%). These results suggest that the suppression of LEURs observed in the SI cortex may result from modulatory actions on subcortical circuits. Major suppression of SEURs, on the other hand, may occur intracortically, with a minor component ocurring subcortically. Thus, VPL thalamus and SI cortex in the rat appear to be differentially subject to movement related modulation of sensory transmission.
Similar content being viewed by others
References
Albe-Fessard D, Gillet E (1961) Convergences d'afferences d'origines corticale et peripherique vers le centre median du chat anesthesie ou eville. Electroenceph. Clin. Neurophysiol 13:257–269
Angel A, Clarke, KA (1975) An analysis of the representation of the forelimb in the ventrobasal thalamic complex of the rat. J Physiol (Lond) 249:399–423
Angel A, Malenka RC (1982) Velocity-dependent suppression of cutaneous sensitivity during movement. Exp Neurol 77:266–274
Chapin JK, Waterhouse BD, Woodward DJ (1981) Differences in cutaneous sensory response properties of single somatosensory cortical neurons in awake and halothane anesthetized rats. Brain Res Bull 6:63–70
Chapin JK, Woodward DJ (1981) Modulation of sensory responsiveness of single somatosensory cortical cells during movement and arousal behaviors. Exp Neurol 72:164–178
Chapin JK, Woodward DJ (1982a) Somatic sensory transmission to the cortex during movement. I. Gating of single cell responses to touch. Exp Neurol 78:654–669
Chapin JK, Woodward DJ (1982b) Somatic sensory transmission to the cortex during movement. II. Phasic modulation over the locomotor step cycle. Exp Neurol 78:670–684
Chapin JK, Lin C-S (1984) Mapping the body representation in the SI cortex of anesthetized and awake rats. J Comp Neurol 229:199–213
Chapin JK, Sorensen SM, Woodward DJ (1986) Acute ethanol effects on sensory responses of single units in the somatosensory cortex of rats during different behavioral states. Pharmacol Biochem Behav 25:607–614
Chapin JK (1987) Modulation of cutaneous sensory transmission during movement: possible mechanism and biological significance. In Wise SP, Evarts EV (eds) Neural and behavioral approaches to higher brain function, Wiley & Sons, New York
Coquery JM (1978) Selective attention as a motor program. In: Requin J (ed) Attention and performance VII. Lawrence Erlbaum, Hilsdale
Coquery JM (1978) Role of active movement in control of afferent input from skin of the cat and man. In: Gordon G (ed) Active touch. Pergammon Press, Oxford, pp 161–179
Coulter JD (1974) Sensory transmission through lemniscal pathway during voluntary movement in the cat. J Neurophysiol 37:831–845
Ebner FF, Armstrong-James MA, Diamond ME (1989) Changes in receptive field properties of rat barrelfield neurons following thalamic lesions. Abstr 19th Ann Mtg Soc Neurosci 1222
Ghez C, Pisa M (1972) Inhibition of afferent transmission in cuneate nucleus during voluntary movement in the cat. Brain Res 40:145–151
Giblin DR (1964) Somatosensory evoked potentials in healthy subjects and in patients with lesions of the nervous system. Ann NY Acad Sci 112:93–102
Gibson JJ (1962) Observations on active touch. Psychol Rev 69:477–491
Hicks SP, D'Amato CJ (1975) Motor-sensory cortex-corticospinal system and developing locomotion and placing in rats. Am J Anat 143:1–42
Knowles SE, Shin C-H, Chapin JK (1985) At what levels of the somatosensory system does MI cortex stimulation modulate cutaneous sensory transmission in the rat? Neuroscience Abstr 264.6
Lee RG, White DG (1974) Modification of the human somatosensory evoked response during voluntary movement. Electroencephalogr. Clin Neurophysiol 36:53–62
Lu SM, Lin C-S (1986) Cortical projection patterns of the medial division of the nucleus posterior thalamus in the rat. Abstr 16th Ann Soc Neurosci Mtg, p 1434
Lund RD, Webster KE (1967) Thalamic afferents from the spinal cord and trigeminal nuclei: an experimental and anatomical study in the rat. J Comp Neurol 130:313–328
Melzack R, Southmayd SE (1974) Dorsal column contributions to anticipatory motor behavior. Exp Neurol 42:274–281
Morrison RS, Dempsey EW (1942) Study of thalamocortical relations. Am J Physiol 135:281–292
Mountcastle VB (1957) Modality and topographic properties of single neurons of cat's somatic sensory cortex. J Neurophysiol 20:408–418
Nelson R (1984) Responsiveness of monkey primary somatosensory cortical neurones to peripheral stimulation depends on “motor-set”. Brain Res 304:143–148
Nelson RJ (1985) Sensorimotor cortex responses to vibrotactile stimuli during the initiation and execution of hand movement. In: Goodwin A, Darian-Smith I (eds). Hand function and the neocortex. Exp Brain Res Suppl 10:59–76
Nelson RJ (1987) Activity of monkey primary somatosensory cortical neurons changes prior to active movement. Brain Res 406:402–407
Nelson RJ, Douglas VD (1989) Set related and pre-movement activity in primary somatosensory cortex differ when monkeys make hand movements in response to visual vs. vibratory cues. Brain Res 484:43–56
O'Brien JH, Rosenblum SM (1974) Contribution of nonspecific thalamus to sensory evoked activity in cat postcruciate cortex. J Neurophysiol 37:430–440
Poggio GF, Mountcastle VB (1960) A study of the functional contributions of the lemniscal and spinothalamic systems to somatic stability: central nervous mechanisms in pain. Bull Johns Hopkins Hosp. 166:266–276
Poggio GF, Mountcastle VB (1963) The functional properties of ventrobasal thalamic neurons studied in unanesthetized monkey. J Neurophysiol 26:775–806
Scheibel ME, Scheibel AB (1967) Structural organization of non-specific thalamic nuceli and their projection toward cortex. Brain Res 6:60–94
Shin H-C, Chapin JK (1989) Mapping the effects of motor cortex stimulation on single neurons in the dorsal column nuclei in the rat: direct responses and afferent modulation. Brain Res Bull 22:245–252
Shin H-C, Chapin JK (1990) Mapping the effects of motor cortex stimulation on single neurons in the VB thalamus in the rat: direct responses and afferent modulation. Brain Res Bull 24:251–265
Shin H-C, Chapin JK (1990) Modulation of afferent transmission to single neurons in the ventroposterior thalamus during movement in rats. Neurosci Lett 108:116–120
Shin H-C, Jin BK, Chapin JK (1989) Somatosensory transmission through the lemniscal system during movement is facilitated and then suppressed: velocity dependence. Abstr 19th Ann Mtg Soc Neurosci 385
Vierek CJ Jr (1978) Interpretations of the sensory and motor consequences of dorsal column lesions. In: Gordon G (ed) Active touch. Pergamon Press, Oxford
Wall PD (1967) The laminar organization of dorsal horns and effects of descending impulses. J Physiol 188:403–423
Wall PD (1970) The sensory and motor role of impulses traveling in the dorsal columns towards cerebral cortex. Brain 93:505–524
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shin, H.C., Chapin, J.K. Movement induced modulation of afferent transmission to single neurons in the ventroposterior thalamus and somatosensory cortex in rat. Exp Brain Res 81, 515–522 (1990). https://doi.org/10.1007/BF02423500
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02423500