Summary
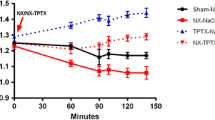
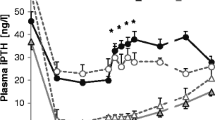
Various investigators have shown that chronic uremia is associated with a normal or exaggerated phosphaturic response to parathyroid hormone (PTH). To explore the relationship between progressive uremia, renal tubular cyclic AMP (cAMP), and inorganic phosphate (Pi) response to PTH and acidosis, in vivo and in vitro experiments were designed in rats with experimental uremia of 4–6 weeks’ duration. Both uremic and pair-fed control rats were treated with 1,25-dihydroxycholecalciferol (1,25(OH)2D3) and/or chronic NH4Cl feeding. Urinary Pi and cAMP and plasma immunoreactive PTH (iPTH) were measured as well as PTH- and NaF-stimulated cAMP from isolated renal tubules. Excretion of cAMP decreased by 30% in uremic as compared to control rats despite a 3-fold rise in Pi excretion. Acidosis superimposed on uremia did not further decrease cAMP excretion, nor did it significantly alter the elevated Pi excretion. 1,25(OH)2D3 treatment of uremic rats further lowered cAMP excretion although Pi excretion rose, hypercalcemia occurred, and plasma iPTH fell. In nonuremic control rats, 1,25(OH)2D3 treatment led to hypercalcemia, a progressive decrease in cAMP, and increase in Pi excretion. Isolated renal tubules from uremic or acidotic uremic rats revealed a 50% reduction in both PTH- and NaF-stimulated cAMP generation compared to control rat renal tubules. This observation was unchanged by 1,25(OH)2D3 treatment. Renal tubules of 1,25(OH)2D3-treated control rats demonstrated a decreased cAMP production in response to both PTH and NaF which was inversely related to the calcium content of the renal tubules. Renal tubular calcium levels of uremic rats, initially 3-fold elevated, also increased during 1,25(OH)2D3 treatment. These results are consistent with the hypothesis that progressive uremia results in a dissociation between PTH, urinary cAMP, and Pi excretion which cannot be explained by either metabolic acidosis or 1,25(OH)2D3 deficiency.
Similar content being viewed by others
References
Slatopolsky, E., Caglar, S., Pennell, J.P., Taggert, D.P., Canterbury, J.L., Reiss, E., Bricker, N.S.: On the pathogenesis of hyperparathyroidism in chronic experimental renal insufficiency in the dog, J. Clin, Invest.50:492–499, 1971
Bricker, N.S.: On the pathogenesis of the uremic state. An exposition of the “trade-off” hypothesis, N. Engl. J. Med.286:1093–1099, 1972
Nagata, N., Rasmussen, H.: Parathyroid hormone and renal cell metabolism, Biochemistry7:3728–3733, 1968
Agus, Z.S., Puschett, J.B., Senesky, D., Goldberg, M.: Mode of action of parathyroid hormone and cyclic adenosine 3′,5′-monophosphate on renal tubular phosphate reabsorption in the dog, J. Clin. Invest.50:617–626, 1971
Chase, L.R., Aurbach, G.D.: Parathyroid function and the renal excretion of 3′,5′-adenylic acid, Proc. Natl. Acad. Sci. U.S.A.58:518–525, 1967
Purkerson, M.L., Rolf, D., Miller, S., Slatopolsky, E., Klahr, S.: Evidence for increased nephron sensitivity to parathyroid hormone (PTH) is renal mass is decreased, Clin. Res.24:591A, 1976 (abstr.)
Beck, N., Kim, H.P., Kim, K.S.: Effect of metabolic acidosis on renal action of parathyroid hormone, Am. J. Physiol.228:1483–1488, 1975
Russell, J.E., Avioli, L.V.: Effect of experimental chronic renal insufficiency on bone mineral and collagen maturation, J. Clin. Invest.51:3072, 3079, 1972
Brumbaugh, P.F., Haussler, D.H., Bressler, R., Haussler, M.R.: Radioreceptor assay 1α, 25-dihydroxyvitamin D3, Science183:1089–1091, 1974.
Forte, L.R., Nickols, G.A., Anast, C.S.: Renal adenylate cyclase and the interrelationship between parathyroid hormone and vitamin D in the regulation of urinary phosphate and adenosine cyclic 3′,5′-monophosphate excretion, J. Clin. Invest.57:559–568, 1976
Chanard, J., Block, R., Purkerson, M., Lewis, J., Klahr, S., Slatopolsky, E.: The effects of colchicine and vinblastine on parathyroid hormone secretion in the rat, Endocrinology101:1792–1800, 1977
Shain, S.A.: Thein vitro metabolism of 25-hydroxy-cholecalciferol to 1,25-dihydroxycholecalciferol by chick renal tubules, J. Biol. Chem.247:4404–4413, 1972
Peytremann, A., Goltzman, D., Callahan, E.N., Tregear, G.W., Potts, J.T., Jr.: Metabolism and biologic activity of proparathyroid hormone and synthetic analogues in renal cortical membranes, Endocrinology97:1270–1280, 1975
Di Belle, F.P., Arnaud, C.D., Brewer, H.B., Jr.: relative biologic activities of human and bovine parathyroid hormones and their synthetic, NH2-terminal (1–34) peptides, as evaluatedin vitro with renal cortical adenylate cyclase obtained from three different species, Endocrinology99:429–436, 1976
Lowry, O.H., Rosenbrough, N.J., Farr, A.L., Randall, R.J.: Protein measurement by the Folin phenol reagent, J. Biol. Chem.193:256–275, 1951
Kepner, B.L., Hercules, D.M.: Fluorometric determination of calcium in blood serum, Anal. Chem.35:1238–1243, 1963
Barzel, U.S., Jowsey, J.: The effects of chronic acid and alkali administration on bone turnover in adult rats, Clin. Sci.36:517–524, 1969
Beck, N., Singh, H., Reed, S.W., Davis, B.B.: Direct inhibitory effect of hypercalcemia on renal actions of parathyroid hormone, J. Clin. Invest.53:717–725, 1974
Aurbach, G.D., Chase, L.R.: Cyclic 3′,5′-adenylic acid in bone and the mechanisms of action of parathyroid hormone, Fed. Proc.29:1179–1182, 1970
Peck, W.A., Carpenter, J., Messinger, K., DeBra, D.: Cyclic 3′,5′-adenosine monophosphate in isolated bone cells: response to low concentrations of parathyroid hormone, Endocrinology92:692–697, 1973
Smith, D.M., Johnston, C., Jr.: Hormonal responsiveness of adenylate cyclase activity from separated bone cells, Endocrinology95:130–139, 1974
Slatopolsky, E., Mercado, A., Morrison, A., Yates, J., Klahr, S.: Inhibitory effects of hypermagnesemia on the renal action of parathyroid hormone, J. Clin. Invest.58:1273–1279, 1976
Beck, N., Webster, S.K.: Effects of acute metabolic acidosis on parathyroid hormone action and calcium mobilization, Am. J. Physiol.230:(1): 127–131, 1976
Mautalen, C., Montoreano, R., Labarrere, C.: Early skeletal effect of alkali therapy upon the osteomalacia of renal tubular acidosis, J. Clin. Endocrinol. Metab.42:875–881, 1976
Chertow, B.S., Baylink, D.J., Wergedal, J.E., Su, M.H.H., Norman, A.W.: Decrease in serum immunoreactive parathyroid hormone in rats and in parathyroid hormone secretionin vitro by 1,25-dihydroxycholecalciferol, J. Clin. Invest.56:668–678, 1975
Henry, H.L., Taylor, A.N., Norman, A.W.: Response of chick parathyroid glands to the vitamin D metabolites 1,25-dihydroxycholecalciferol and 24,25-dihydroxycholecalciferol, N. Nutr.107:1918–1926, 1977
Chertow, B.S., Baker, G.R., Henry, H.L., Norman, A.W.: Inhibition of parathyroid hormone secretion by vitamin D metabolites, Clin. Res.26:412A, 1978 (abstr.)
Canterbury, J.M., Lerman, S., Claflin, A.J., Henry, H., Norman, A., Reiss, E.: Inhibition of parathyroid hormone secretion by 1,25-dihydroxycholecalciferol and 24,25-dihydroxycholecalciferol in the dog, J. Clin. Invest.61:1375–1383, 1978
Oldham, S.B., Smith, R., Hartenbower, D.L., Henry, H.L.: Effects of 1,25-dihydroxycholecalciferol on serum calcium, phosphate and immunoreactive parathyroid hormone in dogs, Adv. Exp. Med. Biol.103: (in press)
Golden, P., Mazey, R., Greenwalt, A., Martin, K., Slatopolsky, E.: Vitamin D: a direct effect on the parathyroid gland? Mineral Electrolyte Metab. (in press)
Scarpelli, D.G., Tremblay, G., Pearse, A.G.E.: A comparative cytochemical and cytologic study of vitamin D induced nephrocalcinosis, Am. J. Pathol.36:331–353, 1960
Ibels, L.S., Alfrey, A.C., Haut, L., Huffer, W.E.: Preservation of function in experimental renal disease by dietary restriction of phosphate, N. Engl. J. Med.298:122–126, 1978
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Russell, J.E., Kleerekoper, M., Slatopolsky, E. et al. Dissociation of renal cyclic AMP and phosphate responses to parathyroid hormone in uremia. Calcif Tissue Int 29, 147–154 (1979). https://doi.org/10.1007/BF02408070
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02408070