Abstract
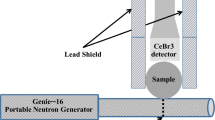
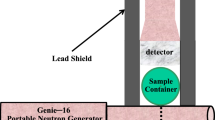
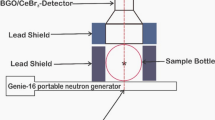
In soil science (ca. 1970), bromide ion (Br−) in various forms (e.g., KBr, NaBr, SrBr2) was introduced as a non-reactive stable tracer in solute transport studies normally moving freely with the flux of water without substantial chemical or physical interactions with the soil. Typically, Br− is extracted from soil and quantified using either a bromide selective electrode (sensitivity is ≈10μg/ml) or by high-performance liquid chromatography (sensitivity is ≈0.010 μg/ml). Where the sensitivity is adequate, the selective conductivity method, which is simple, affordable and fast, is preferred. More recently (ca. 1990), workers have reported that 20% of Br− tracers, at low groundwater pH, may be adsorbed by iron oxides and kaolinite when present in the alluvial aquifer. We investigated the use of Epithermal Neutron Activation Analysis (ENAA) as a means of measuring Br− directly in soil samples without an extraction. ENAA was chosen because of its high theoretical advantage factor over aluminum (i.e. ≈20), the principal interfering soil constituent, calculated for the79Br(n,γ)80Br reaction compared to27Al(n, γ)28Al. Br− was measured (sensitivity is ≈0.050 μg/g) in one gram soil samples from a 5 s irradiation (φepi=2.5·1012 n·cm-2·s-1) using a BN capsule.
Similar content being viewed by others
References
S. J. Smith, R. J. Davis, J. Environ. Quality, 3 (1973) 152.
W. A. Jury, L. H. Stolzy, P. Shouse, Water Resour. Res., 18 (1982) 369.
W. A. Jury, G. Sposito, Soil Sci. Soc. Am. J., 49 (1985) 1331.
T. J. Gish, C. B. Coffman, Transactions of the ASAE, 30 (1987) 1358.
T. J. Gish, A. Shirmohammadi, R. Vyravipillai, B. J. Wienhold, Soil Sci. Soc. Am. J., 59 (1995) 895.
A. P. Vinogradov, Consultants Bureau, 2nd ed., New York 1959.
J. M. Boggs, E. E. Adams, Water Resour. Res., 28 (1992) 3325.
D. C. Adriano, H. E. Doner, A. L. Page (Ed.), Methods of Soil Analysis, Part 2, 2nd ed., 1982.
N. A. Abdalla, B. Lear, Soi. Sci. Plant Anal. 6 (1975) 489.
M. D. Glascock, W. Z. Tian, W. K. Ehmann, J. Radioanal Nucl. Chem., 92 (1985) 379.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kazemi, H.V., Morris, J.S., Anderson, S.H. et al. Measurement of bromide ion used as a solute-transport monitor via epithermal neutron activation analysis. J Radioanal Nucl Chem 235, 249–254 (1998). https://doi.org/10.1007/BF02385970
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02385970