Abstract
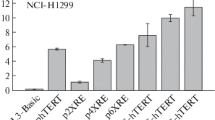
Pristane is a naturally occurring isoprenoid which is believed to be derived from the phytyl moiety of chlorophyll. Thus it is not surprising that pristane is present in many common fruits or vegetables and furthermore can be detected in tissues of fish and mammals. Using the rat as an animal model, pristane can function as a potent tumor promoter. It is conceivable that pristane could play a role in the development of certain malignancies in higher mammals since it is commonly found in the diet. At the molecular level, pristane can induce changes in the plasma membrane, alter the conformation of chromatin, as well as selectively activate gene expression. This study was undertaken to identify specific transcriptional motifs which are responsive to pristane. A transcriptional promoter which contained a cAMP response element (CRE) was consistently stimulated by pristane in several mouse and primate cell lines. A promoter construct which contained a single copy of the TPA response element (TRE) was also activated by pristane but surprisingly a promoter which contained multiple copies of the TRE was not. Activation of the TRE required 10 fold higher concentrations of pristane relative to activation of the CRE. Within two hours after addition of pristane to monkey fibroblasts (CV-1) levels of cAMP were increased more than two fold relative to controls. These data indicated that pristane can increase the level of cAMP in CV-1 cells and consequently stimulate transcriptional promoters which contain a CRE.
Similar content being viewed by others
References
Avigan J, Blumer M: On the origin of pristane in marine organisms. J Lipid Res 9: 350–352, 1968
McKenna EJ, Kallio RE: Microbial metabolism of the isoprenoid alkane pristane. Proc Natl Acad Sci USA 68: 1552–1554, 1971
Bost KL, Garrett LR, Cuchens MA: The role of the Peyer's patch in carcinogenesis. II. 3-methylcholanthrene induced lymphoid malignancies in rat Peyer's patches. Carcinogenesis 7: 1257–1265, 1986
Anderson PN, Potter M: Induction of plasma cell tumors in BALB/c mice with 2, 6, 10, 14 tetramethylpentadecane (pristane). Nature 222: 994–995, 1969
Brodeur BR, Tsang P, Larose Y: Parameters affecting ascites tumour formation in mice and monoclonal antibody production. J Immuno Methods 71: 265–272, 1984
Potter M, Wax JS: Peritoneal plasmacytomagenesis in mice: comparison of different pristane dose regimens. J Natl Cancer Inst 71: 391–395, 1983
Potter M, Sklar MD, Rowe WP: Rapid viral induction of plasmacytomas in pristane-primed BALB/c mice. Science 182: 592–594, 1973
Luquet P, Cravedi JP, Tulliez J, Bories G: Growth reduction in trout induced by naphthenic and isoprenoid hydrocarbons (dodecylcydohexane and pristane). Ecotoxical Environ Safety 8: 219–226, 1984
Hopkins SJ, Freemont AJ, Jayson MIV: Pristane-induced arthritis in BALB/c mice. Curr Top Microbiol Immunol 122: 213–252, 1985
Kripke MC, Weiss DW: Studies on the immune responses of BALB/c mice during tumor induction by mineral oil. Int J Cancer 6: 422–430, 1970
Platica M, Bojko C, Clincea R, Hollander V: Lipopoly-saccharide and pristane-induced peritoneal exudate requirements for plasma tumor cell colony formation. J Natl Cancer Inst 68: 325–328, 1982
Chung J-G, Garrett LR, Byers PE, Cuchens MA: A survey of the amount of pristane in common fruits and vegetables. J of Food Composition and Analysis 2: 22–27, 1989
Avigan J, Milne GWA, Highet RJ: The occurrence of pristane and phytane in man and animals. Biochem Biophys Acta 144: 127–131, 1967
Bly JE, Garrett LR, Cuchens MA: Pristane induced changes in rat lymphocyte membrane fluidity. Cancer Biochem Biophys 11: 145–154, 1990
Garrett LR, Cuchens MA: Pristane induced effects on chromatin of rat lymphoid cells. J of Cellular Biochemistry 45: 311–316, 1991
Garrett LR, Jones C, Cuchens MA: Gene activation by pristane or TPA. Submitted to Chemico Biological Interactions
Castagna M, Takai Y, Kaibuchi K, Sano K, Kikkawa U, Nishizuka Y: Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem 257: 7847–7851, 1982
Montminy MR, Sevarino KA, Wagner JA, Mandel G, Goodman RH: Identification of a cAMP responsive element within the rat somatostatin gene. Proc Natl Acad Sci USA 83: 6682–6686, 1986
Taylor SS, Buechler JA, Yonemoto W: cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem 59: 971–1005, 1990
Roesler WJ, Vanderbark GR, Hanson RW: Cyclic AMP and the induction of eukaryotic gene transcription. J Biol Chem 26: 9063–9066, 1988
Angel P, Imagawa M, Chiu R, Stein B, Imbra RJ, Rahmsdorf HJ, Jonat C, Herrlich P, Karin M: Phorbol ester-inducible genes contain a common cis element recognized by a TPA modulated trans-acting factor. Cell 49: 729–739, 1987
Chiu R, Angel P, Karin M: Jun-B differs in its biological properties from, and is a negative regulator of, c-jun. Cell 59: 979–986, 1989
Graham F, van der Eb A: A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology 52: 456–457, 1973
Jones C: The minimal transforming fragment of HSV-2 can function as a complex transcriptional promoter. Virology 169: 346–353, 1989
Jones C, Delhon G, Bratanich A, Rock D: Analysis of the transcriptional promoter which regulates the latency-related transcript of bovine herpesvirus 1. J Virol 64: 1164–1170, 1990
Sambrook J, Fritsch EF, Maniatis T: Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, 1989, pp 1.21–1.50
Brown BL, Albano JDM, Ekins RP, Sgherzi AM: A simple and sensitive saturation assay method for the measurement of adenosine 3′5′ cyclic monophosphate. Biochemical Journal 121: 561–562, 1971
Beavo JA: Effects of Xanthine derivatives on lipolysis and on adenosine 3′5′ monophosphate phosphodiesterase activity. Molec Pharmacol 6: 597–603, 1983
Montague W, Cook JR: The role of adenosine 3′5′ cyclic monophosphate in the regulation of insulin release by isolated rat islets of langerhans. Biochem J 122: 115–120, 1971
Imagawa M, Chiu R, Karin M: Transcription factor AP-2 mediates induction by two different signal-transduction pathways, protein kinase C and cAMP. Cell 51: 251–260, 1987
Yoshimasa T, Sibley DR, Bouvier M, Lefkowitz RJ, Caron MG: Cross-talk between cellular signaling pathways suggested by phorbol ester adenylate cyclase phosphorylation. Nature 327: 67–70, 1987
Yamamoto KK, Gonzalez GA, Biggs WH, Montminy M: Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature 334: 494–498, 1988
Berkowitz LA, Riabowl KT, Gilman MZ: Multiple sequence elements of a single functional class are required for cyclic AMP responsiveness of the mouse c-fos promoter. Molec and Cellular Biology 9: 4272–4281, 1989
deGroot RP, Auwerx J, Karperien M, Staels B, Kruijer W: Activation of junB by PKC and PKA signal transduction through a novel cis-acting esement. Nucl Acids Res 19: 775–781, 1991
Auwerx J, Sassone-Corsi P: IP-1: A dominant inhibitor of fos/jun whose activity is modulated by phosphorylation. Cell 64: 983–993, 1991
Stein B, Rahmsdorf HJ, Steffen A, Litfin M, Herrlich P: UV-induced DNA damage is an intermediate step in UV induced expression of human immunodeficiency virus type 1, collagenase, c-fos, and metallothionein. Molecular and Cellular Biology 9: 5169–5181, 1989
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lee, S.H., Ackland-Berglund, C.E. & Jones, C.J. The tumor promoter pristane activates transcription by a cAMP dependent mechanism. Mol Cell Biochem 110, 75–81 (1992). https://doi.org/10.1007/BF02385008
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02385008