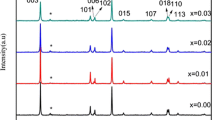
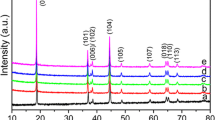
Abstract
We are reporting the synthesis and characterization of solid solutions of the LiNiO2 and LiCoO2 system. Substitution of cobalt for nickel in the LiNi1−yCoyO2 phases provides significant improvements in the two-dimensionality of the crystal lattice and ease the large scale synthesis. This structural effect improves the reversibility of the lithium intercalation-deintercalation process. We have evaluated the vibrational spectra and electrochemical properties of LiNi0.7Co0.3O2 (charge-discharge profiles and cyclic voltammetry) and compared the results with those of the end members, i.e., LiNiO2 and LiCoO2. The local environment of cations against oxygen neighboring atoms has been determined.
Similar content being viewed by others
5. References
M.G. Thomas, W.I.F. David, J.B. Goodenough and P. Groves, Mat. Res. Bull.20, 1137 (1985).
M. Broussely, F. Perton, P. Biensan, J.M. Bodet, J. Labat, A. Lecerf, C. Delmas, A. Rougier and J.P. Peres, J. Power Sources54, 109 (1995).
J.R. Dahn, U. von Sacken, M.W. Juskow and J. Al-Janabi, J. Electrochem. Soc.138, 2207 (1991).
C. Delmas, I. Saadoune and A. Rougier, J. Power Sources43–44, 595 (1993).
L.D. Dyer, B.S. Borie and G.P. Smith, J. Amer. Chem. Soc.76, 1499 (1954).
J.B. Goodenough, D.G. Wickham and W.J. Croft, J. Phys. Chem. Solids5, 107 (1958).
J.N. Reimers, J.R. Dahn, J.E. Greedan, C.V. Stager, G. Liu, I. Davidson and U. von Sacken, J. Solid State Chem.102, 216 (1993).
R. Kanno, H. Kubo, Y. Kawamoto, T. Kamiyama, F. Izumi, Y. Takeda and M. Takano, J. Solid State Chem.110, 216 (1994).
A. Rougier, P. Gravereau, and C. Delmas, J. Electrochem. Soc.143, 1168 (1996).
A. Rougier, I. Saadoune, P. Gravereau, P. Willmann and C. Delmas, Solid State Ionics90, 83 (1996).
J.R. Dahn, U. von Sacken and C.A. Michal, Solid State Ionics44, 87 (1990).
R.K. Moore and W.B. White, J. Am. Ceramic Soc.53, 679 (1970).
M. Inaba, Y. Todzuka, H. Yoshida, Y. Grincourt, A. Tasaka, Y. Tomida and Z. Ogumi, Chem. Lett. 889 (1995).
T. Ohzuku and A. Ueda, J. Electrochem. Soc.141, 2972 (1994).
I. Saadoune and C. Delmas, J. Mater. Chem.6, 193 (1996).
P. Tarte and J. Preudhomme, Spectrochim. Acta26A, 747 (1970).
E. Zhecheva and R. Stoyanova, Solid State Ionics66, 143 (1993).
W. Huang and R. Frech, Solid State Ionics86–88, 395 (1996).
I.F. Chang and S.S. Mitra, Phys. Rev.172, 924 (1968).
P. Tarte, Spectrochim. Acta20, 238 (1964).
P. Tarte, J. Inorg. Nucl. Chem.29, 915 (1967).
P. Hope and B. Schepers, Z. Anorg. Allgem. Chem.295, 233 (1958).
M.H. Brodsky, G. Lucovsky, M.F. Chen, and T.S. Plaskett, Phys. Rev. B2, 3303 (1970).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rougier, A., Nazri, G.A. & Julien, C. Vibrational spectroscopy and electrochemical properties of LiNi0.7Co0.3O2 cathode material for rechargeable lithium batteries. Ionics 3, 170–176 (1997). https://doi.org/10.1007/BF02375613
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02375613